Spencer Fox Eccles School of Medicine
47 Cardiac Adrenergic Stimulation in a Zebrafish Model of Human Atrial Fibrillation
Leah Moellmer; Martin Tristani-Firouzi; Natalia S. Torres; Christopher Kauffman; and Ainsley Hokanson
Faculty Mentor: Martin Tristani-Firouzi (Pediatrics, University of Utah)
Abstract
Atrial fibrillation is a cardiac arrhythmia characterized by an irregular heartbeat in the atrial chambers of the heart. Our previous work has identified the Nuclear Factor of Activated Tcells (NFATC1) as a novel atrial fibrillation susceptibility gene for familial atrial fibrillation (phenotype appearing at <40 years old). NFATC1 is known to be involved in cardiac development, but the link to arrhythmias has not been previously established. Using CRISPR/Cas9 we generated a nfatc1 knock-out (KO) zebrafish transgenic line that showed an increased presence of arrhythmogenic events and a sudden death phenotype in juvenile f ish. Our current objective is to determine whether the absence of nfatc1 influences heart function and response to stress in zebrafish embryos (48- to 120-hours post-fertilization (hpf)). Larvae heart rate was then measured in the presence of 100 μM of isoproterenol (a β-adrenergic agonist) to test their response to adrenergic stimulation. We observed that the heart rates followed the expected increase with age but were not statistically different between genotypes for the same developmental stage. Furthermore, the addition of isoproterenol did not elicit arrhythmias in either genotype. Taken as a whole, these results suggest that nfatc1 does not affect heart rate or response to adrenergic stimulation in early developmental stages. These findings suggest that an impairment in the nfatc1 gene function may be more relevant in the juvenile heart, which is in line with an early onset atrial fibrillation phenotype observed in humans.
Introduction
Atrial fibrillation (AF) is the most common group of cardiac arrhythmias, causing over 28,000 deaths in 2021 (CDC, 2024). This mortality rate is a result of AF causing a significant increase in the risk of stroke, heart failure, and general mortality (Workman, 2010). AF occurrence can be caused by activity in the adrenergic (sympathetic) or cholinergic (parasympathetic) divisions of the autonomic nervous systems (Workman, 2010). The adrenergic division is responsible for the body and heart’s response to stress. Activation of adrenergic systems results in an increase in heart rate, as well as an increase in the force with which the ventricles contract.(Scanlon & Sanders, 2006). Stimulation of the adrenergic division releases adrenergic hormones and catecholamines which bind to the α and β adrenoreceptors that are present in the heart (Workman, 2010).
Isoproterenol is a synthetic agonist that only binds to β receptors (Kuhar et al., 1999). These are the adrenergic receptors most common in the heart. Additionally, a study has found that infusion of isoproterenol produces AF in 5% of patients with no history of AF and 84% of patients with paroxysmal AF (Workman, 2010). This makes isoproterenol an ideal candidate to increase heart rate and potentiate atrial fibrillation in a test species.
Using whole exon sequencing (WES) we have identified NFATC1 as a novel atrial fibrillation susceptibility gene in a family with a familial atrial fibrillation phenotype. Until now, NFATC1 was known to be involved in cardiac development (PMID: 9515963 and PMID: 15166096) but no link to AF was established. Using CRISPR/Cas 9 we developed a nfatc1 KO fish that presents a sudden death and arrhythmogenic phenotype in the juvenile stage (Torres et al., 2023). Because exposure to isoproterenol has been shown to greatly increase the occurrence of AF in subjects who are already prone to arrhythmias, we hypothesize that the use of an adrenergic stimulant will have a differential effect in KO fish, increasing the prevalence of arrhythmias in the KO embryos. This research will advance the knowledge of nfatc1 role in the heart and could strengthen the gene’s link to congenital atrial fibrillation.
Methods
Zebrafish
Zebrafish embryos were produced and raised in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Utah. Embryos were maintained in an animal facility with a controlled light cycle and water temperature. For experiments, 48-, 72-, 96-and 120-hours post fertilization (hpf) embryos were used. This project includes work in wild-type (WT) fish and a CRISPR/Cas9 generated nfatc1 KO zebrafish. When possible, KO fish were compared to their siblings to reduce background genetic variations. For the 48-hpf time point measurements the larvae were mechanically removed from their chorion before being placed in fresh egg water in separate wells.
Imaging
We utilized the transparency of zebrafish larvae to measure heart rate noninvasively. Randomly chosen embryos were placed in egg water in a 24-well plate. The Olympus SZX10 digital imaging microscope was used to record the heart activity in vivo while the embryos were submerged in egg water without the use of anesthesia. Each video was approximately ten seconds in length with a resolution of 600-line pairs per millimeter.
Drug Treatments
We used the beta androgenic agonist isoproterenol to test the embryo’s response to stress. After the heart rate in the control solution was recorded, to ensure optimal control of the drug concentration, the egg water was removed from the well and replaced with egg water containing 100 μM isoproterenol. After two and five minutes in the drug solution, new videos of the embryo heart were recorded. This process was repeated with all developmental stages except at 120-hpf when no videos of the larvae in isoproterenol were recorded. All the experiments were performed at 19.0-21.3 ºC.
Image Processing
Each video was analyzed in the open-source NIH software ImageJ (Schindelin et al., 2012). using the Time Series Analyzer V2 0 plugin (Balaji, 2014). The software recorded intensity values in a region of interest. The resulting trace was then analyzed in Origin® to automatically identify peaks that correlated with a heartbeat. By measuring the time between peaks, we determined the average and instantaneous heart rate.
Statistical Analysis
2-way ANOVA was used to determine whether there was a statistical difference between the base heartrates with the different genotypes at different stages and of fish or between the genotype’s responses to isoproterenol.
Results
Control heart rate was measured in 103 WT fish at 48-hpf, 103 WT fish at 72-hpf, 103 fish at 96-hpf, and 98 WT fish at 120-hpf. In the KO fish, heart rate was recorded in 137 fish at 48hpf, 137 fish at 72-hpf, 137 fish at 96-hpf, and 140 fish at 120-hpf.
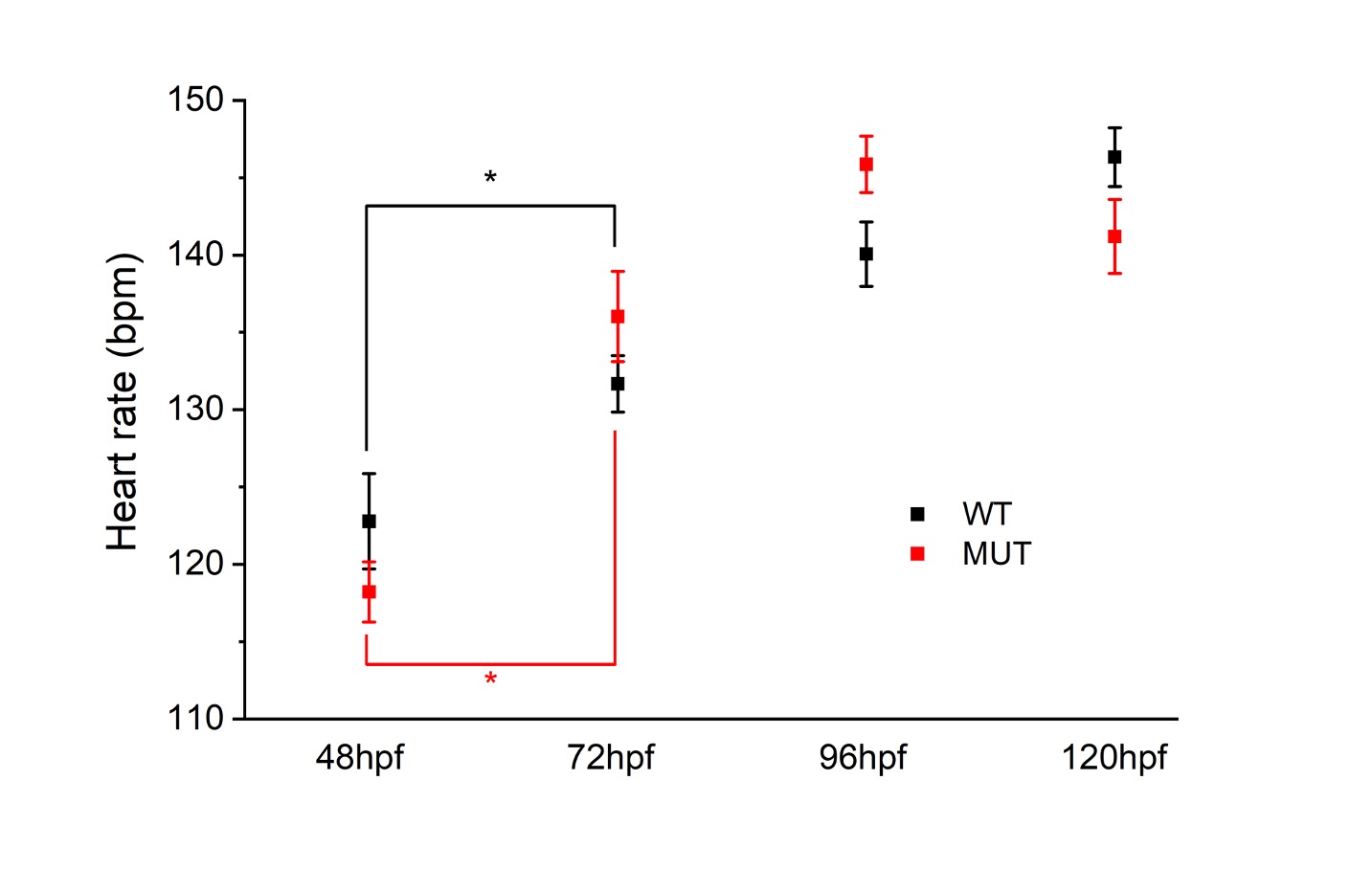
Figure 1. ZF Larae heart rate.
We observed that the heart rates of the nfatc1 KO fish followed the expected increase with age but were not statistically different from the wild-type fish of the same developmental stage (ANOVA, p=0.86 for genotype, p<0.0001 for hpf).
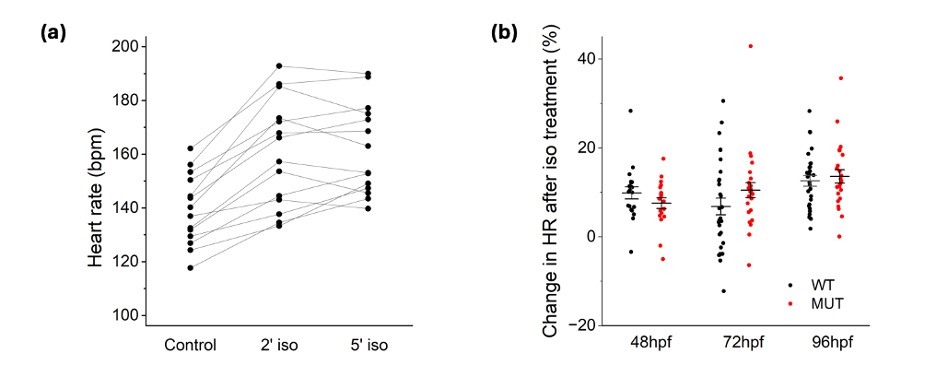
Figure 2. Isoproterenol effect on heart rate.
Heart rates were measured in 38 WT 96-hpf larvae and 72 KO 96-hpf larvae in control solution and after 2- and 5-minutes exposure to 100 μM isoproterenol. The response to the drug was time-dependent (a), reaching the peak effect at 2 minutes. This timepoint was therefore used to measure the increase in heart rate and occurrence of arrhythmias in the larvae. An increase in heart rate after isoproterenol exposure was measured at 48-hpf, 72hpf, and 96-hpf. The 120-hpf timepoint was excluded, as the increased movement of the f ish at this age made the heartrate readings unreliable. The increase in heart rate was measured in 36 WT larvae at 48-hpf, 38 WT larvae at 72-hpf, 38 WT larvae at 96-hpf, 72 KO larvae at 48-hpf, 72 KO larvae at 72-hpf, and 72 KO larvae at 96-hpf. Exposure to isoproterenol increased the heart rate in all experimental groups, but this increase was independent of genotype (b) (ANOVA, p=0.55).
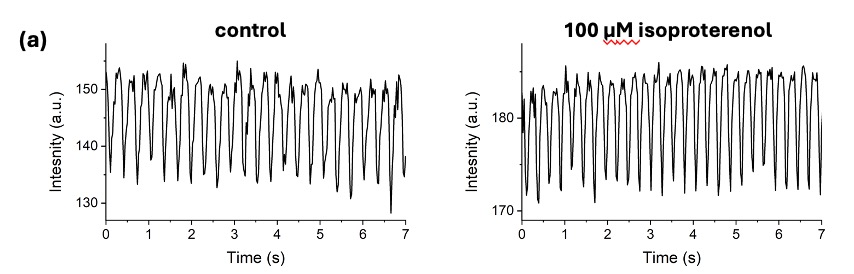
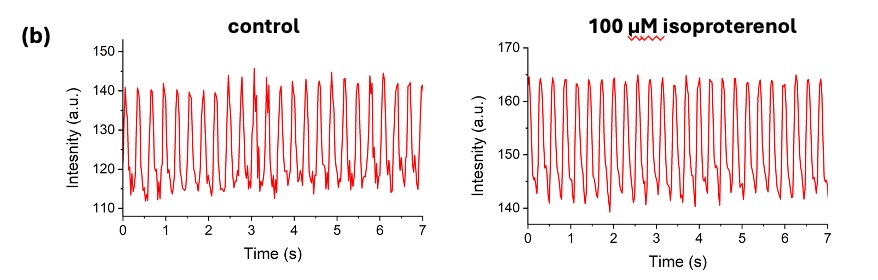
Figure 3. Addition of isoproterenol did not elicit arrhythmias in either genotype.
Representative traces before and after 2 minutes of isoproterenol treatment in WT (a, black) and MUT (b, red) fish show that neither group showed the presence of arrhythmias after the addition of isoproterenol.
Discussion
The purpose of the present study was to determine whether the absence of the nfatc1 gene in larval zebrafish would affect their heart rate at baseline or when experiencing adrenergic stress. Previous studies have found that juvenile zebrafish without the nfatc1 gene are more prone to arrhythmogenic events. However, it had not been established if this trend would be present in larval-stage zebrafish. Heart rate in nfatc1 KO fish has also not been established, nor has its relationship to the wild-type fish. The results of this study suggest that heart rate and response to adrenergic stimulation are not affected by the removal of the nfatc1 gene in early developmental stages. The heart rate of the WT and KO larvae were not statistically different from one another at the same age post-fertilization. The increase in the larvae’s heart rate was also not different between genotypes. No presence of arrhythmias was seen in either genotype, suggesting that adrenergic stress does not increase the prevalence of arrhythmogenic events at this developmental stage. AF in humans typically manifests later in life (>70 years of age), as wear on the heart increases (Brundel et al., 2022). In the case of early onset AF, as seen in the family with mutations to the NFACT1 gene, AF is still rare before middle age. The findings suggest that an impaired gene function may be more relevant in the juvenile heart, a result that aligns with an early onset atrial fibrillation phenotype observed in humans.
Bibliography
Alhayek, S., & Preuss, C. V. (2024). Beta 1 Receptors. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532904/
Balaji, J. (2014). Time Series Analyzer V2 0 plugin (Version 3.0) [Computer software].
Brundel, B. J. J. M., Ai, X., Hills, M. T., Kuipers, M. F., Lip, G. Y. H., & de Groot, N. M. S. (2022). Atrial fibrillation. Nature Reviews. Disease Primers, 8(1), 21. https://doi.org/10.1038/s41572-022-00347-9
CDC. (2024, May 20). About Atrial Fibrillation. Heart Disease. https://www.cdc.gov/heartdisease/about/atrial-fibrillation.html
Kuhar, M. J., Couceyro, P. R., & Lambert, P. D. (1999). α- and β-Adrenergic Receptors. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Lippincott-Raven. https://www.ncbi.nlm.nih.gov/books/NBK28138/
Scanlon, V. C., & Sanders, T. (2006). Essentials of Anatomy and Physiology. F. A. Davis Company. http://ebookcentral.proquest.com/lib/utah/detail.action?docID=3009577
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., & Cardona, A. (2012). Fiji: An open-source platform for biological-image analysis. [Computer software]. Nature Methods, 9(7), 676–682. doi:10.1038/nmeth.2019
Torres, N. S., Acuna, A., Kauffman, C. A., & Tristani-Firouzi, M. (2023). NFATC1 modulation of atrial excitability in zebrafish. Biophysical Journal, 122(3), 164a–165a. https://doi.org/10.1016/j.bpj.2022.11.1040
Vornanen, M., & Hassinen, M. (2015). Zebrafish heart as a model for human cardiac electrophysiology. Channels, 10(2), 101–110. https://doi.org/10.1080/19336950.2015.1121335
Vornanen, M., & Hassinen, M. (2015). Zebrafish heart as a model for human cardiac electrophysiology. Channels, 10(2), 101–110. https://doi.org/10.1080/19336950.2015.1121335
Media Attributions
- 137489262_figure1(1)
- 146879582_figure2(1)
- 146879585_figure3a
- 146881378_figure3b

