College of Pharmacy
62 Synthetic Glycosaminoglycan-Based Treatment Development for Head and Neck Squamous Cell Carcinomas
JaMarah Davis; Abby Pulsipher; and Prince Minkag
Faculty Mentor: Abby Pulsipher (Pharmaceutics & Pharmaceutical Chemistry, University of Utah)
Introduction
Head and neck squamous cell carcinomas (HNSCCs) represent a significant global health concern, with approximately 890,000 new cases and 450,000 related deaths reported annually. These malignancies originate in the mucosal epithelium of the throat, oral cavity, paranasal sinuses, and nasal passages, and they present with a broad spectrum of clinical manifestations, leading to varying prognoses. Standard treatment options, including surgery, radiotherapy, chemotherapy, and adjuvant immunotherapy, form the backbone of HNSCC management. However, despite advancements in these therapeutic modalities, more than 65% of patients still experience metastatic or recurrent disease, underscoring the urgent need for more effective treatment strategies.
A promising focus of recent research has been the CXCL12/CXCR4 signaling pathway, which has emerged as a critical driver of HNSCC progression. This pathway plays a pivotal role in tumor cell survival, proliferation, invasion, resistance to chemoradiation, and recurrence. Heparin, traditionally employed as an anticoagulant, has demonstrated potential as an anti-tumor agent by inhibiting this signaling axis. Nevertheless, the clinical application of heparin in oncology is complicated by its short half-life and significant anticoagulant activity, which pose concerns regarding safety and limit its therapeutic utility in HNSCC.
Considering these challenges, this project seeks to develop and evaluate synthetic heparin-like glycosaminoglycans that selectively target the CXCL12/CXCR4 pathway while minimizing the anticoagulant effects associated with natural heparin. These synthetic compounds are designed to disrupt key pro-tumor processes, offering a novel adjuvant therapeutic strategy for patients with advanced and metastatic HNSCC. The successful development of these agents could significantly improve treatment outcomes, providing new hope for patients facing limited therapeutic options.
Methods
Synthetic schematics were employed to generate synthetic heparin-like glycosaminoglycans based on hyaluronic acid (HA) with minimal anticoagulant properties and potent activity against the CXCL12/CXCR4 pathway. Glycosaminoglycans were synthesized and characterized using Fourier transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance (1H NMR). The binding affinity of compounds to CXCL12 and the effect of compounds on blood coagulation were assessed using a sandwich immunoassay and prothrombin time (PT) and activated partial thromboplastin time (aPTT) tests, respectively. To evaluate statistical significance among the compounds, a one-way ANOVA was conducted, followed by Dunnett’s multiple comparison tests (p<0.05). Heparin and HA served as controls for glycosaminoglycans.
Results
Novel glycosaminoglycans were synthesized by crosslinking HA with a known chemical crosslinking agent, resulting in a modified product designated as HA10B. The HA10B intermediate was subsequently subjected to chemical sulfation, with varying degrees of sulfation ranging from 3 to 10. The focus of this study is on the compound with a sulfation level equivalent to 7, referred to as SXHA7 and later named BJC42. To ensure purity, the BJC42 compound underwent dialysis for 72 hours to remove any residual impurities.
Following the synthesis and sulfation of BJC42, the compound underwent detailed characterization. Analyses using FTIR (Figure 1) and 1H NMR (Figure 2) confirmed that both cross linking and sulfation were successful, establishing BJC42 as a promising candidate for further study. With these results in hand, the focus shifted to assessing BJC42’s binding affinity to the CXCL12 pathway, a critical target in this research.
Figure 1 & 2
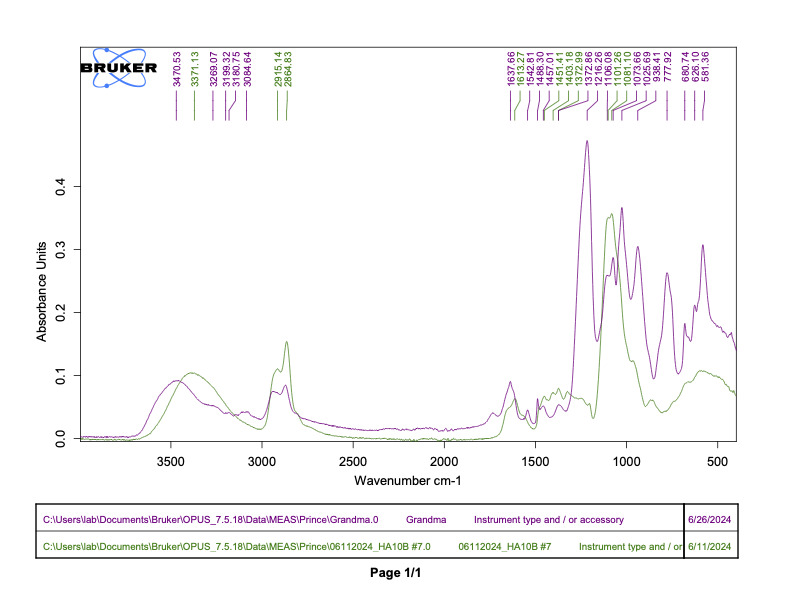
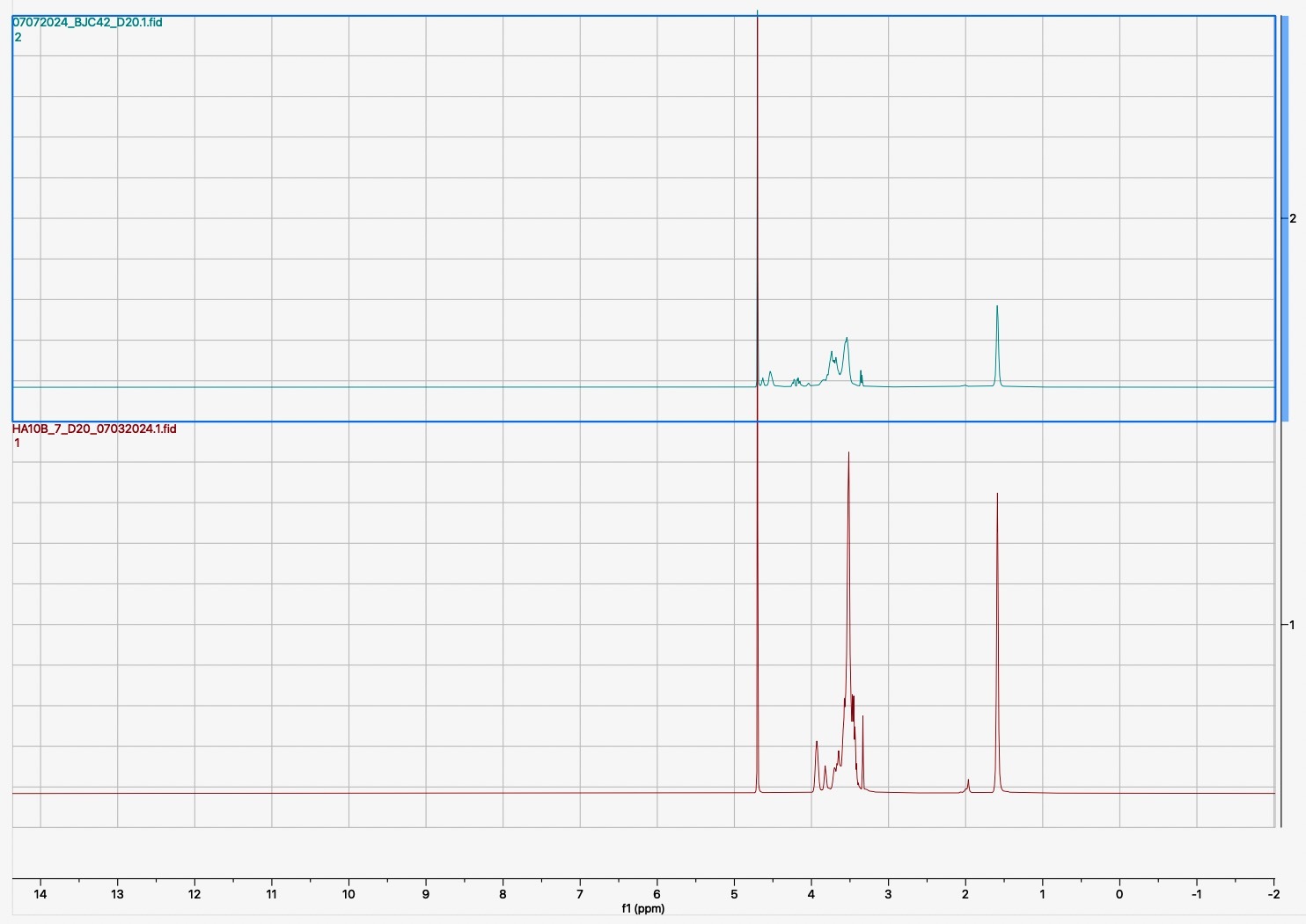
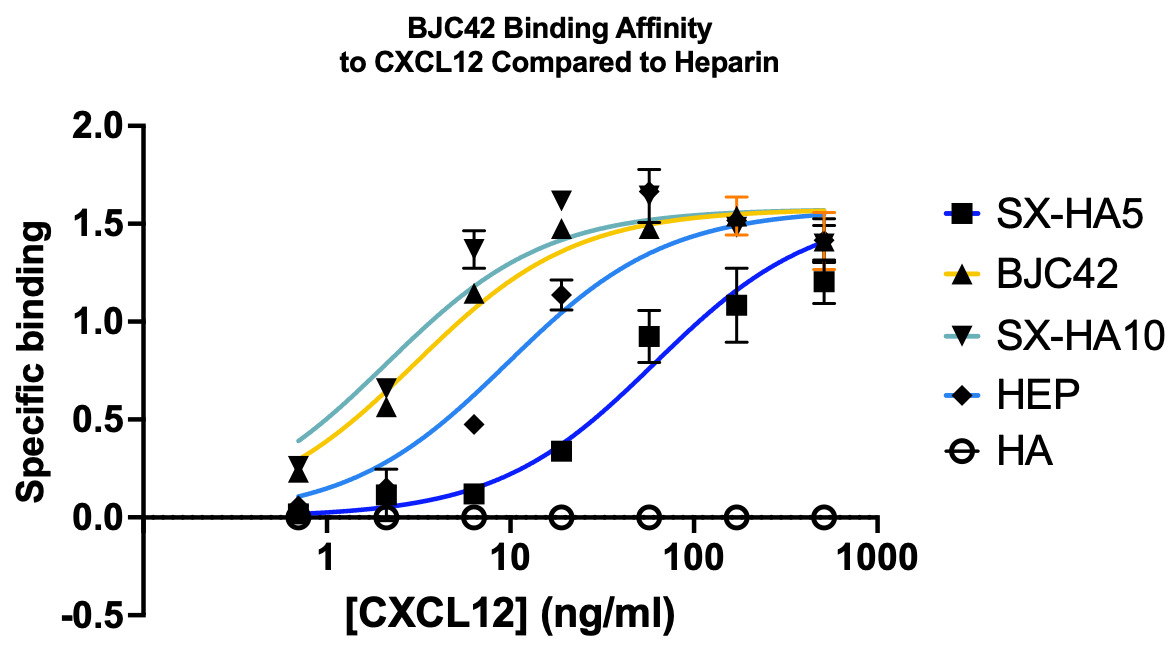
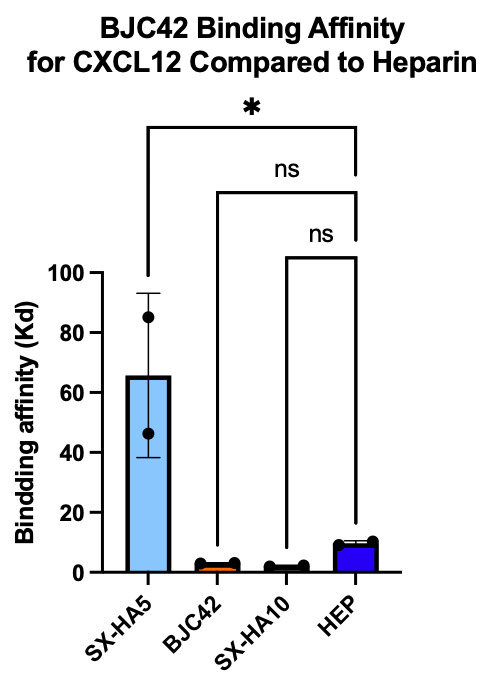
Binding affinity was evaluated using a sandwich ELISA method on a 96-well plate. The data revealed that BJC42 exhibited a high binding affinity to CXCL12 across a range of concentrations, performing closely to that of heparin, the foundation for this pathway targeting (Figures 3 & 4).
Figure 3 & 4
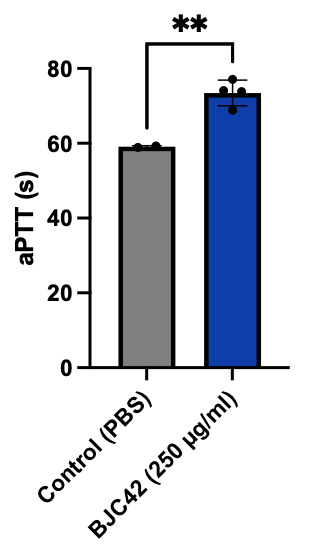
Encouraged by these binding results, the next phase of the study aimed to evaluate the anticoagulant activity of BJC42. A coagulometer was used to measure PT (Figure 5) and aPTT (Figure 6) blood clotting time of human plasma. The results showed that BJC42 did indeed have anticoagulant properties, but these effects were concentration-dependent. In the PT assay, BJC42 was tested at concentrations of 250 μg/ml and 125 μg/ml. At 125 μg/ml, BJC42’s coagulation behavior closely resembled that of the control (phosphate-buffered saline/PBS), with no significant differences observed. However, in the aPTT assay, conducted at a concentration of 250 μg/ml, BJC42 displayed a somewhat significant difference in coagulation time compared to the control, suggesting that its anticoagulant activity is sensitive to concentration levels.
Figure 5 & 6

Conclusion
The successful synthesis and sulfation of the synthetic heparin-like glycosaminoglycan, BJC42, yielded notable results. The compound demonstrated high binding affinity for CXCL12 without notable anticoagulant activity, positioning it as a leading candidate in the study of synthetic glycosaminoglycans for potential treatment of HNSCCs. These results indicate that BJC42 merits further investigation as a possible adjuvant therapy for HNSCCs, suggesting it could be an important addition to current treatment approaches.
Future Directions
Further characterization of BJC42 is essential before advancing to preliminary safety screenings. This characterization will involve determining the molecular weight, polydispersity, sulfur content, and the degree of crosslinking. BJC42, along with other promising compounds, will then undergo in vitro therapeutic feasibility assessments using HNSCC models. These assessments will include CXCL12-CXCR4 and VEGF-C/VEGFR3 competitive binding assays, as well as evaluations of CXCL12/CXCR4-mediated HNSCC cell invasion and proliferation, and VEGF-C/VEGFR3-mediated tumor proliferation and lymphangiogenesis.
Following the completion of in vitro studies, the most promising compounds will be selected for in vivo therapeutic assessments using a patient-derived xenograft HNSCC mouse model. Preclinical studies will focus on evaluating therapeutic efficacy of lead candidates in reducing tumor volume, as well as pharmacokinetic and pharmacodynamic investigations. These studies are designed to establish the safety of the compounds before progressing to clinical trials.
Acknowledgements
This work was supported by SPUR from the Office of Undergraduate Research at the University of Utah awarded to JaMarah D. Davis. This opportunity was provided by the Research Experiences to Advance the Careers of HBCU Undergraduates at the University of Utah initiative, co-funded by the School of Medicine and Huntsman Cancer Institute. This work was also supported by an Incentive Seed Grant awarded to Abigail Pulsipner of the office of the vice President for Research and School of Medicine, at the University of Utah.
Bibliography
Dhull AK, Atri R, Dhankhar R, Chauhan AK, Kaushal V. Major risk factors in head and neck cancer: A retrospective analysis of 12-year experiences. World J Oncol. 2018;9(3):80-4.
Ma SN, Mao ZX, Wu Y, Liang MX, Wang DD, Chen X, Chang PA, Zhang W, Tang JH. The anti- cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14(1):118-28.
Shi Y, Riese Dj and Shen J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020;11:574667.
Su, JL., Yen, CJ., Chen, PS. et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96:541-5.
Media Attributions
- 137489262_06262024_grandma_vrs_ha10b_7 (1)
- Screenshot
- 146881378_12252a57-38b5-40e6-bccc-76f564cda180
- 146879585_4aedb574-9fa5-4b40-a8fb-1f37cfe45ca6 (1)
- 146881870_img_8161
- 146882801_img_5266

