College of Health
32 Effects of Exercise on GABA Levels in the Aging Brain
Ryder Robins; Bradley King; Genevieve Albouy; Abigail White; Anke Van Roy; and Adriana Coletta
Faculty Mentor: Bradley King (Health and Kinesiology, University of Utah)
Background
Aging is associated with substantial declines in cognitive and motor functioning1,2. These declines may be attributed, in part, to age-related decreases in the levels of gamma-aminobutyric acid (GABA) – the brain’s primary inhibitory neurotransmitter – in task relevant brain regions3. GABA plays an important role in the excitation/inhibition balance essential for optimal brain functioning, and as a result, age-associated decreases in GABA have been linked to known decrements in cognition, motor learning, and sensory processing. As exercise has shown promise to mitigate such aging-related decrements4,5,6, one candidate mechanism suggests that it does so by increasing brain GABA levels7,8,9. In this proof-of-concept study, we aim to investigate the extent to which an acute bout of high intensity aerobic exercise can modulate brain GABA levels in healthy older adults. We use magnetic resonance spectroscopy (MRS) to quantify GABA in the primary motor cortex (M1) and the hippocampus (HC) – two brain regions selected for their roles in motor and cognitive functioning, respectively. We hypothesize that high intensity aerobic exercise, as compared to a control (rest) intervention, will increase regional GABA concentrations in older adults, mitigating known age-related decreases in brain GABA levels3.
Methods
The preliminary sample consisted of four apparently healthy, right-handed males between 72-74 years old. Exclusion criteria included known neurological, psychological, and psychiatric conditions as well as contraindications to MRI or high intensity exercise. Participants underwent three sessions over a period of approximately three weeks (Figure 1). The first session consisted of a 6-minute walk test to estimate VO2max10 and measurements of height, weight, resting heart rate (HR), and blood pressure (see Table 1 for participant characteristics). In the second and third sessions, participants completed an exercise or control intervention (order counterbalanced across participants). The exercise intervention consisted of a high-intensity interval training (HIIT) regimen in which periods of high intensity exercise (85-95% of peak heart rate) were alternated with periods of active recovery (50-75% of peak heart rate). For the control intervention, participants sat quietly for a duration equivalent to the HIIT. To measure GABA levels, a 3T Siemens scanner was used to perform GABA-edited MRS immediately pre and post each intervention. A high-resolution T1 structural image at the beginning of each scan was referenced to place voxels over the left M1 (3 x 3 x 3cm3) and the left HC (4 x 2.5 x 2.5cm3), which are displayed in Figures 2a and 2b, respectively. Data were acquired using the HERMES11 sequence (320 averages M1; 400 averages HC) and analyzed using Gannet 3.3.212. GABA concentrations were computed relative to creatine. Blood draws were taken pre and post intervention to measure blood-based markers of plasticity (BDNF and AEA; data not included here). To minimize within-participant session differences and dietary confounds, participants were instructed to maintain similar diet intake and exercise regimens the day prior to each scanning session. Participants also completed an overnight fast prior to being provided with a fat-free standardized breakfast the morning of each scanning session.
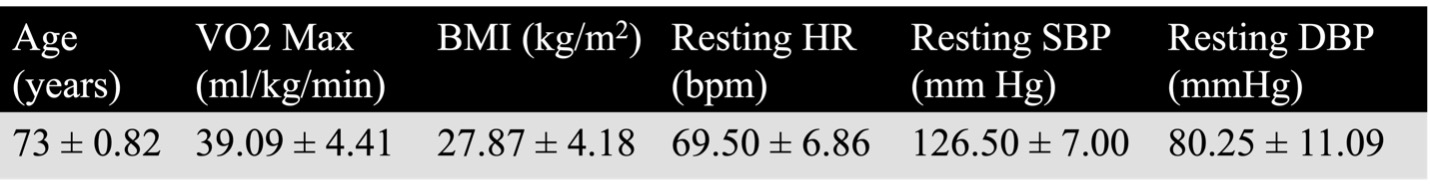
Table 1. Participant characteristics; values represent means ± standard deviation. BMI: body mass index; HR: heart rate; SBP and DBP: systolic and diastolic blood pressure, respectively
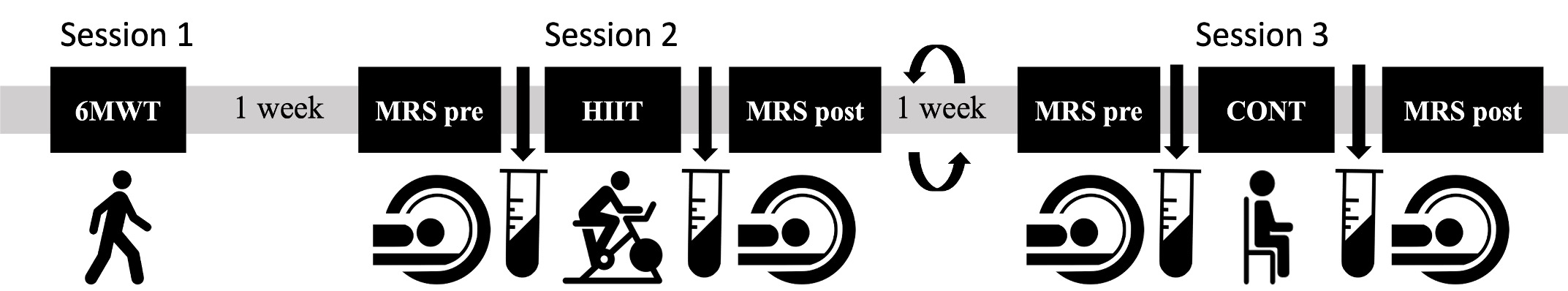
Figure 1. Outline of experimental protocol. 6MWT: 6-minute walk test; MRS: magnetic resonance spectroscopy scan of GABA; HIIT: high intensity interval training; CONT: control (rest) intervention.
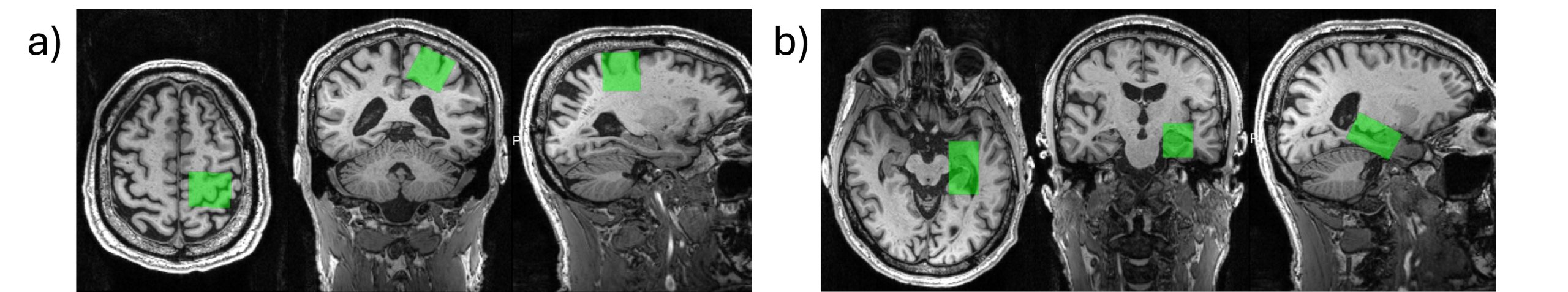
Figure 2. Voxel placement over the left M1 (a) and the left hippocampus (b).
Results
The HIIT intervention was successful in increasing the HR of every participant, although some participants fell short of the 90-95% intensity goal (Figure 3). For participant safety, HR within 80-90% maximum was accepted. As expected, there were minimal changes in HR throughout the rest condition. The exercise intervention resulted in a small increase in GABA levels in M1 (Figure 4a). However, this increase does not appear to differ from the control intervention. In our preliminary sample of older adults, exercise resulted in a small decrease in hippocampal GABA levels while the control intervention resulted in a slightly larger decrease (Figure 4b). In an adjacent and nearly identical study in young adults (n=13), the same exercise intervention resulted in a small but negligible increase in hippocampal GABA levels compared to the control intervention (Figure 4c). It is worth noting that there was substantial loss of hippocampal GABA data in both younger and older adult subjects due an unreliable MR signal scanning deep in the brain as well as artifacts caused by participants moving in the scanner.
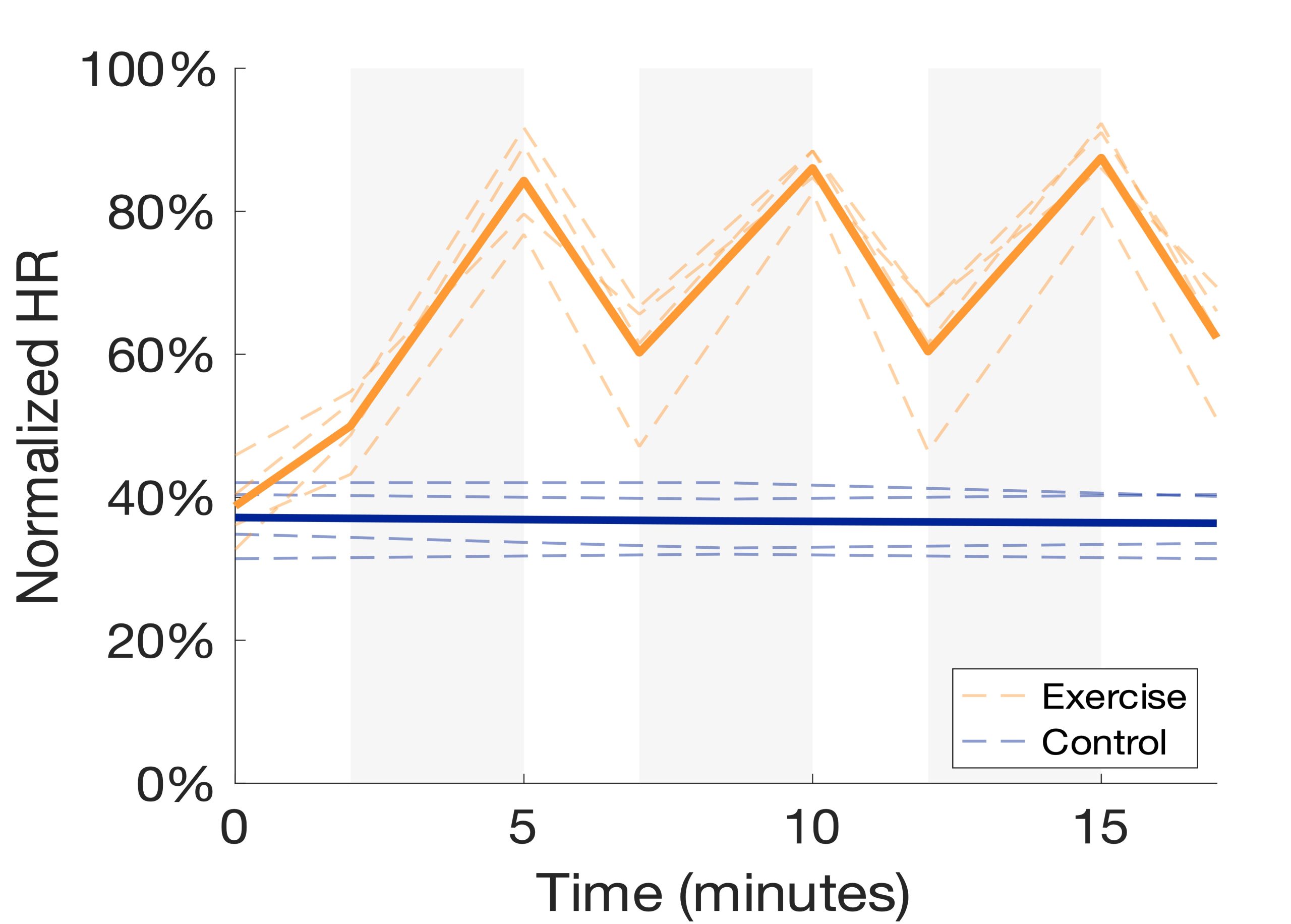
Figure 3. HR intensity normalized to age-predicted maximums and plotted throughout exercise (orange) and control (blue) interventions. Gray shaded regions represent high intensity intervals of the HIIT intervention. Bolded lines represent group averages.
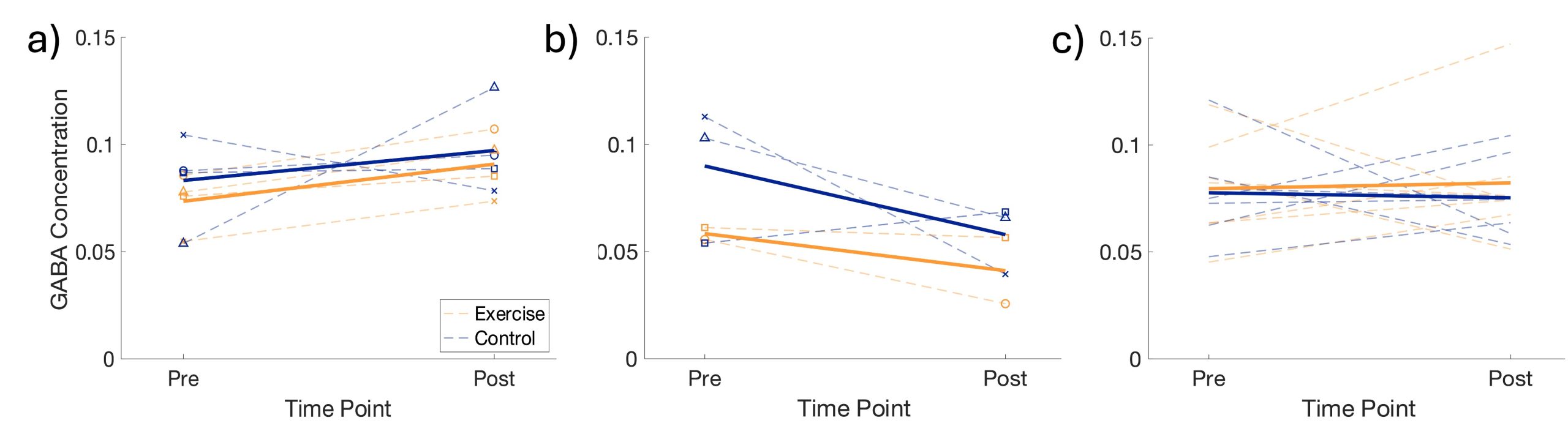
Figure 4. GABA levels in M1 of older adults (a), the HC of older adults (b), and the HC of younger adults (c) pre and post the exercise (orange) and control (blue) interventions. Bolded lines represent group averages.
Conclusions and Future Directions
The HIIT intervention successfully modulated HR compared to the control intervention. However, preliminary data suggest that GABA levels in M1 and the hippocampus did not increase as a result of exercise. More data are required to elucidate trends in the data and draw definitive conclusions. Data loss from the hippocampus was a substantial barrier in this research but shows promise in future studies thanks to higher powered MR scanners and improved GABA detection sequences emerging in the literature13,14. Next, this study will utilize blood samples to analyze levels of BDNF and AEA while working towards completing the collection goal of 15 older adult participants.
Footnotes
1. Deary, I.J., Corley, J., Gow, A.J., Harris, S.E., Houlihan, L.M., Marioni, R.E., Penke, L., Rafnsson, S.B., and Starr, J.M. (2009). Age-associated cognitive decline. British Medical Bulletin 92, 135152. https://doi.org/10.1093/bmb/ldp033.
2. Seidler, R. D., Bernard, J. A., Burutolu, T. B., Fling, B. W., Gordon, M. T., Gwin, J. T., Kwak, Y., & Lipps, D. B. (2010). Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neuroscience and Biobehavioral Reviews 34(5), 721–733. https://doi.org/10.1016/j.neubiorev.2009.10.005.
3. Gao, F., Edden, R.A.E., Li, M., Puts, N.A.J., Wang, G., Liu, C., Zhao, B., Wang, H., Bai, X., Zhao, C., et al. (2013). Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. https://doi.org/10.1016/j.neuroimage.2013.04.012.
4. Voss, M.W., Vivar, C., Kramer, A.F., and van Praag, H. (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences 17, 525–544. https://doi.org/10.1016/J.TICS.2013.08.001.
5. McSween, M.-P., Coombes, J.S., MacKay, C.P., Rodriguez, A.D., Erickson, K.I., Copland, D.A., and McMahon, K.L. (2019). The Immediate Effects of Acute Aerobic Exercise on Cognition in Healthy Older Adults: A Systematic Review. Sports Medicine 49, 67–82. https://doi.org/10.1007/s40279-018-01039-9.
6. Erickson, K.I., Voss, M.W., Prakash, R.S., Basak, C., Szabo, A., Chaddock, L., Kim, J.S., Heo, S., Alves, H., White, S.M., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 108, 3017–3022. https://doi.org/10.1073/pnas.1015950108.
7. Coxon, J.P., Cash, R.F.H., Hendrikse, J.J., Rogasch, N.C., Stavrinos, E., Suo, C., and Yücel, M. (2018). GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. The Journal of Physiology 596, 691–702. https://doi.org/10.1113/JP274660.
8. Maddock, R.J., Casazza, G.A., Fernandez, D.H., and Maddock, M.I. (2016). Acute Modulation of Cortical Glutamate and GABA Content by Physical Activity. The Journal of Neuroscience: the official journal of the Society for https://doi.org/10.1523/JNEUROSCI.3455-15.2016.
9. McGregor, K., Champion, G., Nocera, J., Novak, T., Goodwin-Hamel, C., Mammino, K., Walters, C., and Krishnamurthy, L. (2022). Changes in Functional GABA Levels in Motor Cortex in Older Adults. Archives of Physical Medicine and Rehabilitation 103, e130. https://doi.org/10.1016/j.apmr.2022.08.778.
10. Mänttäri, A., Suni, J., Sievänen, H., Husu, P., Vähä-Ypyä, H., Valkeinen, H., Tokola, K. and Vasankari, T. (2018). Six-minute walk test: a tool for predicting maximal aerobic power (VO2 max) in healthy adults. Clinical Physiology and Functional Imaging 38, 10381045. https://doi.org/10.1111/cpf.12525.
11. Saleh, M.G., Oeltzschner, G., Chan, K.L., Puts, N.A.J., Mikkelsen, M., Schär, M., Harris, A.D., and Edden, R.A.E. (2016). Simultaneous edited MRS of GABA and glutathione. Neuroimage 142, 576–582. https://doi.org/10.1016/j.neuroimage.2016.07.056.
12. Edden, R. A., Puts, N. A., Harris, A. D., Barker, P. B., & Evans, C. J. (2014). Gannet: A batchprocessing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging 40(6), 1445–1452. https://doi.org/10.1002/jmri.24478.
13. Lim, S. I., & Xin, L. (2022). γ-aminobutyric acid measurement in the human brain at 7 T: Short echo-time or Mescher-Garwood editing. NMR in Biomedicine 35(7), e4706. https://doi.org/10.1002/nbm.4706.
14. Moser, P., Hingerl, L., Strasser, B., Považan, M., Hangel, G., Andronesi, O. C., van der Kouwe, A., Gruber, S., Trattnig, S., & Bogner, W. (2019). Whole-slice mapping of GABA and GABA+ at 7T via adiabatic MEGA-editing, real-time instability correction, and concentric circle readout. Neuroimage 184, 475–489. https://doi.org/10.1016/j.neuroimage.2018.09.039.
Media Attributions
- 137489262_table1
- 146879582_expprotocol
- 146879585_voxels
- 146881378_exercise
- 146881870_gaba

