The Periodic Table
Objective 2.5
2.5.1 Classify the arrangement of elements in a periodic table.
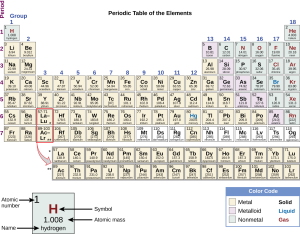
Elements are listed in the periodic table in order of atomic number (number of protons and electrons in a neutral atom). As we progress along a row from left to right, and between rows from top to bottom, the atomic numbers increase in order. The periodic table consists of a series of boxes or blocks with essential information about the element: the atomic number, the chemical symbol, and either the mass number or atomic mass.
When writing an element with its atomic number or mass number outside of the periodic table, however, scientists put the atomic number as a subscript before the symbol and the mass number as a superscript before the symbol. For example, hydrogen (symbol H) is atomic number 1 so it is shown as:
1H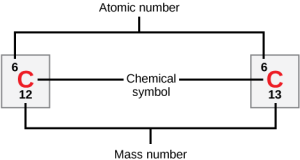
The atomic mass of hydrogen can be 1, 2, or 3, so these three isotopes (protium, deuterium, and tritium, respectively) are written:
1H, 2H, 3H
In 1869, Mendeleev discovered that known elements could be arranged in a periodic table. If one arranges elements in numerical order by atomic number, leaving blanks in some rows, elements with similar properties can be grouped into columns.
![]()
Elements in the same column (group) of the periodic table have similar chemical properties, just like days of the week have the same properties.
Elements in the same column (group) of the periodic table have the same number of valence electrons, which is what gives them similar chemical properties. Remember chemistry is what happens when electrons interact.
These are called groups and are numbered from 1 to 18. For example, in the first column, sodium (Na) and potassium (K) have many chemical properties in common, such as which elements they like to combine with. Sometimes, the groups have names:
Group 1 (H, Li, Na, K, and chemically similar elements) are called the alkali metals.
Group 2 (Be, Mg, Ca, and chemically similar elements) are called the alkaline earth metals.
Group 17 (F, Cl, Br, I) are the halogens.
Group 18 (He, Ne, Ar, Kr, Xe, Rn) are the noble gases.
Metals are on the left 2/3 of the periodic table (yellow blocks). Non-metals are on the far right side of the table (green blocks). For example, calcium (Ca) is the most common metal in the human body (atomic number 20, 1.5% of body mass) while oxygen is the most common non-metal (atomic number 8, 65.0%).
There are elements that share metal and non-metal properties and are called metalloids (lilac blocks). The only metalloid atoms found in trace quantities in the human body are boron (B) and silicon (Si). The metalloids form a dividing line between the metals below and to the left and the non-metals above and to the right.
The far-right column (group 18) contains atoms that do not combine with other atoms. For this reason, they are called noble gases. (Remember that oxygen combines with itself to form O2 molecules, oxygen gas. Argon is 1% of the atmosphere and is the most common of the noble gases, but it won’t even combine with itself, so argon gas is simply Ar.) Because the noble gases don’t combine with anything, they are not present in the human body. Still, they have their uses; we will revisit the noble gases in Objective 6.
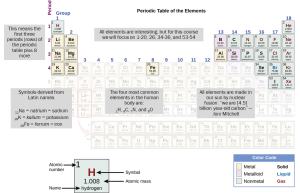
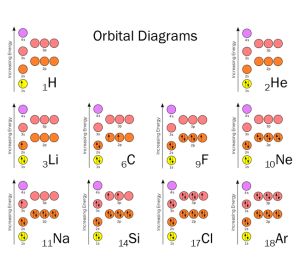
For this course, you need to know the names and symbols (but not the atomic numbers or masses) for the elements shown below.
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulfur (S)
- Chlorine (Cl)
- Argon (Ar)
- Potassium (K)
- Calcium (Ca)
- Iron (Fe)
- Selenium (Se)
- Bromine (Br)
- Krypton (Kr)
- Iodine (I)
- Xenon (Xe)
Note that in most cases, the symbols are the first, first and second, or first and third letters in the element name. The exceptions are sodium (Na), potassium (K), and iron (Fe). This is because in the Renaissance when these elements were named, they were given Latin names (natrium, kalium, and ferrum, respectively).
A blood condition is called by the suffix –emia. Hypernatremia is too much sodium in the blood, while hyponatremia is too little. Hyperkalemia is too much potassium in the blood, while hypokalemia is too little.
Now that we know the names of the elements, let’s turn to their arrangement in the periodic table. The arrangement depends on their chemical properties, so that elements with similar chemical properties are stacked on top of each other in groups. How do we describe the chemical properties of an element?
If we learn to interpret the orbital diagrams that were introduced in Objective 4, then we can use them to figure out chemical properties. An example is potassium.
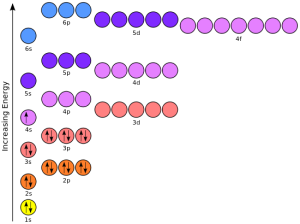
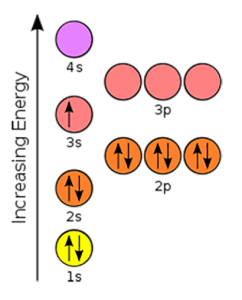
The right figure shows all the available orbitals. But for the first 20 elements, the ones we’re focused on, we only need the 1s, 2s, 2p, 3s, 3p, and 4s orbitals. Those are the ones we’ll focus on in order to simplify these diagrams. We show those in the below figure.
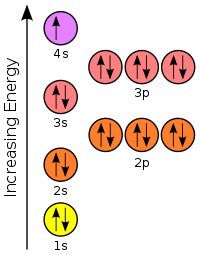
Another way of representing orbitals is shown here.
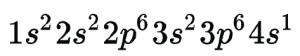
Notice the diagram with colored circles and this shorthand notation have the same basic information, but we feel it’s easier for beginners to understand the diagrams so we’ll tend to use these going forward in this Unit.
Atoms collide with the right orientation and velocity. Upon colliding, they either break or form chemical bonds.
Chemical reactions, the breaking and forming of chemical bonds, are the basis for all biological systems. Remember that only the electrons are involved in chemical reactions.
Atoms form chemical bonds by giving, taking, or sharing electrons. Remember from Objective 4 that atoms are most stable when their outer shells are filled. As an example, take the noble gas krypton (18Kr). Because it’s a noble gas, we know it has an optimal number of electrons (i.e. the electron orbitals are arranged in the lowest possible energy state). A chlorine atom (17Cl) can achieve a stable 18 electrons by adding one to become Cl–. A potassium (19K) atom can reach 18 electrons by giving one away to become K+.
Another way that atoms can achieve filled shells, and the lower energy state that results, is by sharing electrons. The sharing of electrons produces very stable interactions between atoms, what is called a covalent chemical bond. For example, one carbon atom (C4+) shares 4 electrons with two oxygen (O2–) atoms to form carbon dioxide, CO2. We will discuss sharing or trading of electrons to form chemical bonds in the Energy & the Chemical Bond objective.
The number shown here in superscript following the element symbol is referred to as the valence of the atom. In general, the valence is the same as the number of electrons that will be taken to form anions (expressed as a negative number) or the number of electrons which will be given away to form cations (expressed as a positive number).
The uppermost orbital, in terms of energy, is called the valence orbital and participates in chemical reactions. The bond formed between two or more atoms that share electrons is called a covalent bond.
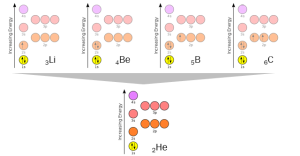
Let’s look at the elements with 3, 4, 5, or 6 electrons in the neutral atom: 3Li, 4Be, 5B, and 6C. Each of these can reach the stable electron configuration represented by 2He by shedding electrons: 3Li wants to shed 1 electron, 4Be wants to shed 2 electrons, 5B wants to shed 3 electrons, and 6C wants to shed 4 electrons (but, importantly, it can also pick up 4, as we’ll see later). The position of carbon in the orbital diagrams, and therefore in the periodic table, is what makes this element the basis for life on Earth.
11Na, 12Mg, and 13Al also want to shed electrons to achieve the stable configuration represented by 10Ne. This is shown in the electron orbital diagrams and Bohr atoms represented here.
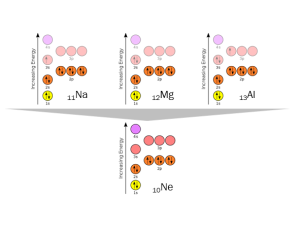
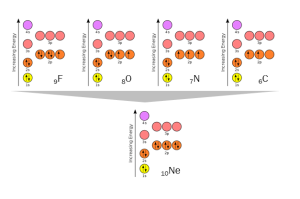
Now you should be comfortable with the concept of shedding electrons to reach stable configurations of electrons. Can an element gain electrons to achieve the same goal? Sure. Taking 10Ne as the ideal again, we see how 6C, 7N, 8O, and 9F gain 4, 3, 2, or 1 electron (respectively) to achieve the stable 10Ne configuration, with the 1s, 2s, and 2p orbitals completely filled with electron spin pairs.
Periodicity in the Periodic Table: Valence
This diagram shows the relationship between sodium’s valence, its electron configuration in the shell model, and its electron configuration in the orbital model.
Sodium is atomic number 11. That means there are 11 protons in the nucleus. This number cannot change by chemical means. What can change is the number of electrons.
 In the neutral sodium atom, there are 11 protons (packets of positive charge) and 11 electrons (packets of negative charge). +11 –11 = 0.
In the neutral sodium atom, there are 11 protons (packets of positive charge) and 11 electrons (packets of negative charge). +11 –11 = 0.
In the sodium ion, sodium reaches its low-energy configuration by giving away one electron. That lone electron is in the outermost shell in the shell model (top) and in the 3s orbital in the orbital model (bottom). Sodium ions still have those 11 protons (+11) but now have only 10 electrons (–10): +11 –10 = +1.
We say the valence of sodium is +1.
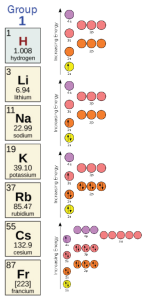
We’ve already seen how different groups in the periodic table want to take, or give away, electrons to reach a stable configuration. For example, hydrogen (H), lithium (Li), sodium (Na), and potassium (K) in Group 1, at the far left of the periodic table, all want to give away one electron to reach a stable configuration. Compare their orbital diagrams: each has one “extra” electron to give away.
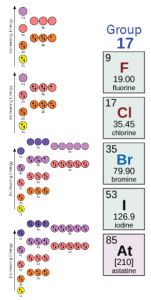
Near the right edge of the periodic table, in Group 17, we find the halogens, which are highly chemically reactive. This is because they like to steal electrons to complete their top orbitals and reach a low energy state.
Note how, in each case, there is one “open parking spot” where a single electron (arrow) can be added to complete the orbital and achieve that low energy state.
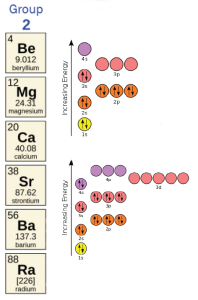
Magnesium (Mg) and calcium (Ca) are important elements in the human body, regulating a wide variety of cellular processes. Calcium is also an important component of bones (Unit 9).
They are both in group 2, and they both have similar chemistry. How many electrons do they want to give away? Magnesium has 12 protons (+12) and when ionized has 10 electrons (–10), so it has a valence of +12 –10 = +2. Calcium has 20 protons (+20) and when ionized has 18 electrons (–18) so it also has a valence of +20 –18 = +2.
The Canvas course includes a worksheet and formative exercises that allow you to see these patterns more clearly.
The tendency of Group 1 elements to reach a low energy state when there is one more proton than electron is indicated as +1. The tendency of Group 17 elements to have one fewer proton, since they take an extra electron, is indicated as –1.
Remember that electrons have a negative charge, so for example, fluorine has 9 packets of positive charge (+9) and when it is ionized, has 10 packets of negative charge (–10) and so overall has a charge of +9 –10 = –1.
Sodium has 11 packets of positive charge (+11) and when it is ionized, also has 10 packets of negative charge (–10) and so overall has a charge of +11–10 = +1. The red numbers at the top of each column (group) indicate the most common valence, and therefore the chemical properties of that group.
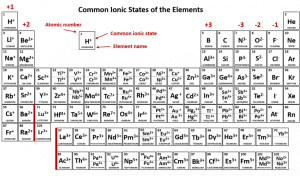
It’s relatively simple to figure out how the elements in Groups 1-2, and 13-17 are going to act, and that’s why those have big red numbers at the top. (Carbon, in group 14, is not labeled but it either gives away 4 electrons (C4+) or takes 4 electrons (C4–) since it is between the +3 and –3 columns of the periodic table. The ability of carbon to either take or give away electrons is the basis for the chemistry of living things, organic chemistry, which we will discuss in Unit 3 and for the rest of the course.
In the middle of the periodic table, we find the transition elements, where the filling of electron shells becomes quite complicated. As you can see by squinting and looking at the valence numbers for each block, these elements have valences that are all over the place and don’t follow any regular pattern. The only one we’re concerned with is iron (26Fe) because it likes to hold onto oxygen. Iron exists in a +2 and +3 form (ferrous and ferric, respectively) and the +2 (ferrous) form is found caged in a molecule called heme which acts as an oxygen carrier in the body.
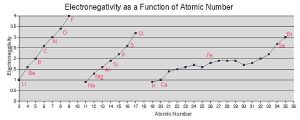
Periodicity in the Periodic Table: Electronegativity
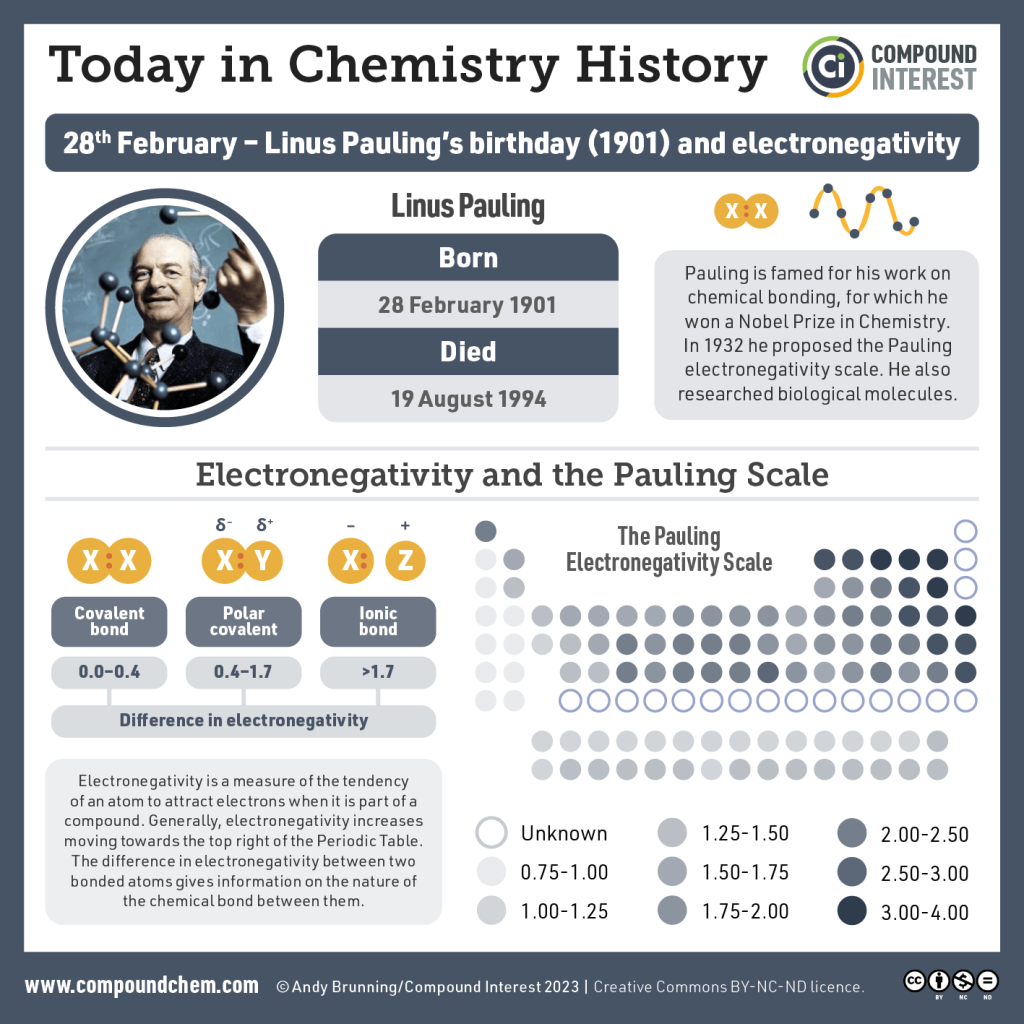
Electronegativity is another chemical property that helps us sort elements in the periodic table. If we remove group 18, the noble gases, because they don’t participate in chemistry, then electronegativity increases as we move towards the higher group numbers (17, on the right) and upper periods (rows). Thus, fluorine (9F), the lightest element in group 17, has the highest electronegativity of any element. At the other end of the periodic table, potassium (19K) in group 1 has one of the lowest electronegativities of all the elements.

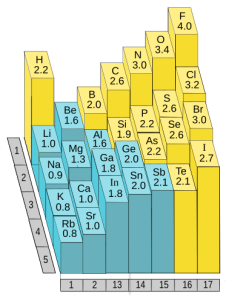
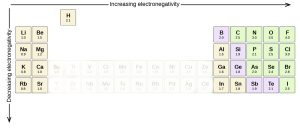
In orbital terms, this means there is a low-energy orbital which is almost, but not quite, full. This is why group 16 and 17 elements have the highest electronegativities.
Note we are not concerned with exact numbers, nor do you have to learn them. Different sources give different numbers. Rather, we want you to know the most electronegative elements (F > O > Cl > N > Br > S) because their electronegativity gives them a unique chemistry that we’ll focus on in later objectives.
Media Attributions
- U02-027 periodic table block © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U02-027a elements in one column graphic © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-028 periodic table © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. is licensed under a CC BY (Attribution) license
- U02-029 periodic table highlights © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U02-030 Orbital representation diagram potassium © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-031 Orbital diagram 19 potassium – Copy © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-032 orbital formula potassium © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-033 Valence Orbitals Page 2-27 © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-035 Page 28 Orbital Helium © CK-12 Foundation adapted by Kenneth Pringle is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-036 Orbitals Neon 1 © CK-12 Foundation adapted by Kenneth Pringle is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-038 valence electrons neon 2 © CK-12 Foundation adapted by Kenneth Pringle is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-039 orbital diagram sodium © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-040 group 1 orbital diagrams © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-042 group 17 orbital diagrams © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-044 group 2 orbital diagrams © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-046 Common-ionic-states-of-the-elements-768×451 © Abozenadah, H., Bishop, A., Bittner, S. and Flatt, P.M. is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- U02-047 02-28-Linus-Paulings-birthday © Brunning, Andy is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-048 linus pauling quote © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-049 Electronegativity hutchins mod © すじにくシチュー is licensed under a CC0 (Creative Commons Zero) license
- CNX_Chem_01_03_PeriodSimp © UC Davis adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U02-051 Electronegativity_as_a_function_of_atomic_number hutchins mod with element symbols © Zullo, Joseph and Levine, Thomas adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license

