The Cross-Bridge Cycle
Objective 10.6
10.6.1 List the sequence of events from an increase in calcium in the muscle cell to contraction of the muscle.
The action potential in a muscle initiates contraction through a series of chemical reactions and protein interactions. Pay attention to the names of the key players in this objective and the role they play in shortening the sarcomere.
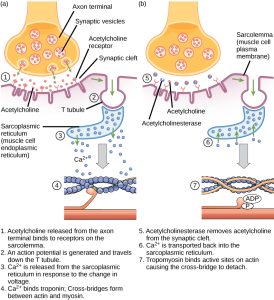
The calcium released from the sarcoplasmic reticulum interacts with a special protein, found only in muscle cells, called troponin. When calcium binds to troponin, it changes its shape, and pushes another specialized protein, tropomyosin. Tropomyosin normally covers the myosin binding sites on the actin molecule, preventing myosin heads from attaching and pulling in on the actin.
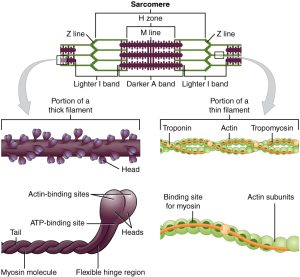
When calcium is present, troponin shoves tropomyosin, moving it away from the binding sites, which allows myosin to bind to the actin. This binding of myosin to actin starts a process called the cross-bridge cycle. It is called this because a link between myosin and actin forms, then breaks, then forms again, over and over.
It’s a cross-bridge cycle, so we can start anywhere, but let’s start at the top of these diagrams, at 12 o’clock on an imaginary clock face.
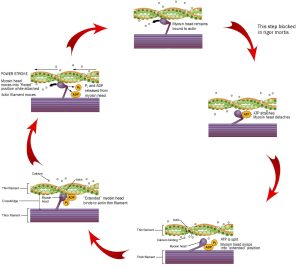
We start as the previous cross-bridge cycle has just completed, with the myosin head bound to the actin filament.
ATP is required for this process. When ATP binds to the myosin head, it releases the actin filament. People who are recently dead have no available ATP in their muscles. Because of this, we remain stuck at 12 o’clock in this diagram, with myosin bound irreversibly to actin. This biochemical condition is called rigor mortis when we see it in an entire human body.
At 3 o’clock, ATP binds to the myosin head and it releases the actin filament.
At 5 o’clock, ATP is split. The energy is stored in the myosin head for later use. Right now, the splitting of adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and inorganic phosphate (Pi) results in a conformational change in the myosin head, converting it from a “flexed” (bent) position to an “extended” (straight) position.
At 8 o’clock, calcium comes in and causes a conformational change in troponin, which shoves the tropomyosin, exposing the myosin-binding site on actin molecules in the thin filament. Myosin, still in its “extended” position, grabs onto the actin thin filament.
At 10 o’clock, ADP and Pi leave the myosin head. Now we use the previously-stored energy from ATP. In the power stroke, the myosin head (still bound to actin) changes shape as ADP and Pi leave. As myosin heads “flex” (bend), the thick filament myosin heads slide the thin filament actin molecules along, generating force as the sarcomere shortens.
Notice the actin filament moving to the left in this diagram as the purple thick filament stays in one place. This is the connection to the sliding filament model seen earlier (Objective 4). 10-26
If the signal from the lower motor neuron stops, the calcium is actively pumped back into the sarcoplasmic reticulum. When this happens, troponin and tropomyosin return to their original location blocking actin binding sites. Without anything to bind to, myosin pulls away from the actin and the proteins mentioned earlier allow for the sarcomere to return to its normal, resting length.
Media Attributions
- U10-004 1003_Thick_and_Thin_Filaments © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U10-026 cross-bridge cycle © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U10-027 Figure_38_04_06f © LibreTexts is licensed under a CC BY-SA (Attribution ShareAlike) license

