Special Properties of Water
Objective 2.11
2.11.1 Describe the special properties of water: hydrogen bonding and surface tension.
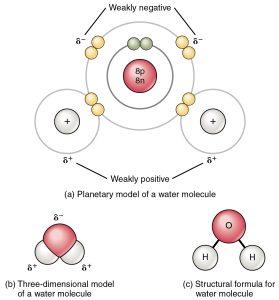
Water has special hydrogen bonding properties.
Water is thought of as a near-universal solvent. A wide variety of substances dissolve in water.
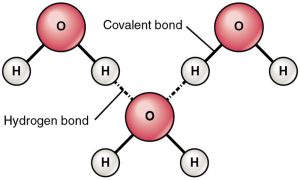
A property called surface tension results from the hydrogen bonds between water. Water molecules are strongly attracted to each other and seem to “pull” together like an elastic sheet. Surface tension can cause delicate baby lungs to collapse if we don’t administer a surfactant—a biological soap that breaks hydrogen bonds. Surface tension also leads to capillary action, when blood seems to “leap” into a narrow hematocrit tube in defiance of the Law of Gravity. Surfactants disrupt the hydrogen bonds between water molecules and thereby prevent capillary action.
Most substances are denser as solids than liquids. Water is different. Water is less dense as a solid than a liquid—ice floats. Frozen water does not allow its molecules to rotate and so it does not “pack” as tightly as liquid water where the water molecules can rotate and find a close-packing configuration.
Ionic bonds are easily disrupted by water, which of course has charges of its own in its polar covalent bonds. This is why salt (NaCl) easily dissolves in water; as it does so, the Na+ ion surrounds itself with water molecules and the δ– O end of water orients towards the positively charged Na+. Similarly, Cl– attracts the δ+ H molecules in water and Cl– is surrounded by a shell of water with its molecules oriented the other way.
These oriented water molecules surrounding ions in solution are called hydration shells. The hydration shells of Na+ and Cl– are discussed and shown in Objective 13.
When water (H2O, which is also written HOH) is split to form H+ and OH– ions, the resulting charged ions contribute to pH, a property that we will discuss next.
Media Attributions
- U02-073 polar covalent bonds in a water molecule © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U02-074 Hydrogen_Bonds_Between_Water_Molecules-01 © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license

