Solutions
Objective 2.13
2.13.1 Compare the molecular properties of a solution, colloid, and suspension.
2.13.2 Explain how to prepare a solution of a given molarity.
2.13.3 Describe the property of osmolarity.
We need a simple way to describe how many of these submicroscopic particles are found hanging out in between water molecules. This is called the concentration of the solution. We also want to know the concentration of particles in water, because particles take up room and thereby reduce the number of water molecules per unit volume. This is called the osmolarity of the solution, which is when we don’t care what type of particles we’re dealing with, just how much room they occupy.
Solutions, Colloids & Suspensions
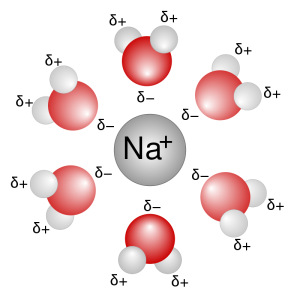
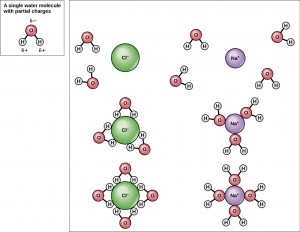
A solution consists of small molecule-sized particles surrounded by water. For example, when NaCl is dissolved in water, Na+ and Cl– in the ionic crystal are each separated and surrounded by the opposite partial charges (a group of δ– O atoms in the H2O molecule pointing towards the Na+ and a bunch of δ+ H atoms in the H2O molecule pointing towards each Cl– ion). Similar hydration shells are formed around other + or – ions. These individual ions or polyatomic ions (see Objective 14) are too small to alter the path of visible light, so the solution appears clear (that is, salt water and pure water look the same)
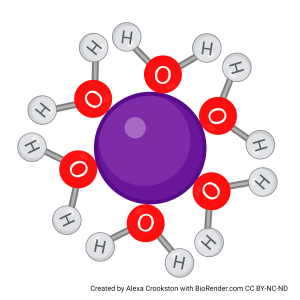 This same process of surrounding molecules with hydration shells occurs whenever a particle (such as the purple cation here) is capable of interacting with the partial charges of water.
This same process of surrounding molecules with hydration shells occurs whenever a particle (such as the purple cation here) is capable of interacting with the partial charges of water.
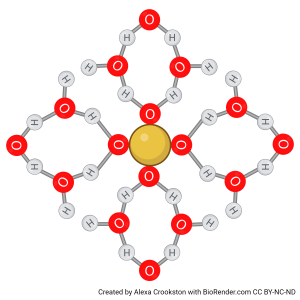 For example, the hydration shell formed by the polyatomic ion SO42– is shown below. The yellow S atom is surrounded by four red O atoms, which in turn attract the partial positive charges of multiple water molecules.
For example, the hydration shell formed by the polyatomic ion SO42– is shown below. The yellow S atom is surrounded by four red O atoms, which in turn attract the partial positive charges of multiple water molecules.
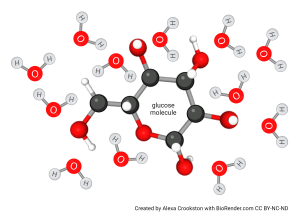 In a solution of small molecules such as sugar, the sugar or other small molecules remain intact and becomes surrounded by a hydration shell formed by hydrogen bonds. Note this can only happen if the small molecule is capable of forming hydrogen bonds (i.e. has plentiful O, N, or S). For example, table sugar (sucrose) is C12H22O11; with 11 oxygens capable of hydrogen bonding. It is very soluble in water. Oleic acid, a component of olive oil, is C18H34O2; with only 2 oxygens capable of hydrogen bonding. Because it is predominantly C and H (i.e. a hydrocarbon), it is not very soluble in water. We will look at this further in Unit 3.
In a solution of small molecules such as sugar, the sugar or other small molecules remain intact and becomes surrounded by a hydration shell formed by hydrogen bonds. Note this can only happen if the small molecule is capable of forming hydrogen bonds (i.e. has plentiful O, N, or S). For example, table sugar (sucrose) is C12H22O11; with 11 oxygens capable of hydrogen bonding. It is very soluble in water. Oleic acid, a component of olive oil, is C18H34O2; with only 2 oxygens capable of hydrogen bonding. Because it is predominantly C and H (i.e. a hydrocarbon), it is not very soluble in water. We will look at this further in Unit 3.
As for individual ions surrounded by a hydration shell, a water-soluble, small molecule surrounded by a hydration shell does not scatter light. A solution of table sugar appears clear, the same as pure water.
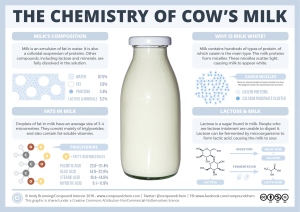
A colloid is formed when the molecules are big enough to scatter light. For example, the proteins in milk are large enough molecules that they can interfere with the travel of visible light. For this reason, even skim milk appears cloudy.
When particles are the size of cells, and can be separated by a tabletop centrifuge, we call the resulting mixture a suspension. Blood is a suspension; as we will see in Unit 15, if you centrifuge blood the red and white blood cells separate from the colloid (large proteins) in a salt solution. The colloidal proteins in an NaCl solution are called blood plasma.
Molarity: Measuring the Strength of a Solution with One Component
In the emergency department, if tissue-typed, cross-matched blood or fresh-frozen plasma is not available, and we need to quickly and safely replace the volume of blood lost in trauma, we use sterile saline, which is 0.9% NaCl. That means that 0.9 g of NaCl have been dissolved in 100 g of water. (The Latin word per cent, symbolized %, literally means “out of 100”.) Because 100 g of water has a volume of 100 mL (which is the same as 100 cm3 or 100 cc), to make a 0.9% NaCl solution, someone at the factory added 0.9 g of NaCl to 100 mL of pure water.
Scientists use 10.94 g sucrose in 100 mL water (10.94% sucrose) for similar purposes, as discussed below.
For most applications in biochemistry and medicine, we don’t want to know the percentage of NaCl or sucrose. We want to know how many molecules of NaCl or sucrose are in 1 liter (1 L) of solution. How is this even possible?
Luckily, Amadeo Avogadro worked this out for us. He developed a relationship between the molecular weight of a substance and the number of molecules of the same substance. If we simply add together the atomic masses of Na (22.99 g) and Cl (35.45 g), we get the molecular weight of NaCl (22.99 g + 35.45 g = 58.44 g).
That means if we weigh out 58.44 g of NaCl, we know exactly how many molecules of NaCl we have. That number is called a mole and is exactly 6.02 × 1023.
Avogadro’s number, or 6.02 × 1023, is a number without a unit, a so-called dimensionless number. That seems strange at first until we realize we’ve come across dimensionless numbers before.
For example, a dozen is dimensionless; whether we get a dozen eggs or a dozen bagels or a dozen pencils, we have 12 objects.

If we get a gross of pencils or firecrackers, we have 12 × 12 = 144 of those objects. A mole works just the same. A mole of NaCl is 58.44 g, and contains 6.02 × 1023 NaCl molecules. A mole of sucrose is 342.3 g and contains 6.02 × 1023 molecules. A mole of hydrogen gas (H2) is 2.002 g (twice the atomic weight of hydrogen) and contains 6.02 × 1023 molecules of hydrogen gas. And so forth.
Now to make a solution where we know the number of molecules in a given volume, all we need do is add a defined mass of the substance (such as 58.44 g NaCl) and bring the total volume to 1 L with water. Now we have a 1 molar (abbreviated 1 M) NaCl solution. (Another, identical, unit is moles/L.) Each liter of this solution contains 6.02 × 1023 NaCl molecules. A liter of a 1 M sucrose solution contains 6.02 × 1023 sucrose molecules. It’s a lot easier to measure the volume of a molar solution than it is to count molecules.
Osmolarity: Measuring the Strength of a Solution with Many Components
One more twist. For blood replacement, and many other purposes, it’s not important to know what molecules are in solution, just how many there are. That’s why you can replace blood with 0.9% NaCl (0.15 M NaCl): there are as many particles in a liter of this solution as there are in a liter of blood.
When we count particles regardless of what the particles are, the quantity is called the osmolarity (Osm) of the solution. Blood plasma is 0.3 Osm/L. 0.9% NaCl is 0.3 Osm/L. 10.94% sucrose is 0.3 Osm/L.

The tube on the left has a low water concentration. The tube on the right has a high water concentration.
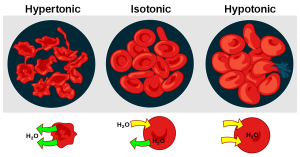
We can think of this two ways. One way is to look at the concentration of water, which tells us which way water is moving. Since water is the only substance that can move, this is what we’ll consider in the diagrams at the bottom of this image. At left, we see a situation where water concentration is higher inside the cell than outside; the green arrows show water moving out. In the center, the water concentration is equal on both sides. In this case, water still moves, but for every water molecule that moves in (yellow arrow) there is a water molecule that moves out (green arrow). Finally, at bottom right, we see a situation where the water concentration is higher outside than inside the cell. In that case, water moves into the cell, as shown by the yellow arrows.
The complication arises because of the names people put on these three situations. They called them by the particle concentration, which is the opposite of the water concentration. The word “tonic” means “strength” (as in muscle tone), so the particle strength is high in a hypertonic solution and low in a hypotonic solution.
When the particle concentration is high, the water concentration is low, and vice versa. So taking these left to right again, but looking at the top labels and images of red blood cells, we have:
- hypertonic (high particle concentration and low water concentration outside the cell, low particle concentration and high water concentration inside, water moves out of the cell)
- isotonic (same particle and water concentration inside and outside, no net water movement)
- hypotonic (low particle concentration and high water concentration outside the cell, high particle concentration and low water concentration inside, water moves into the cell)
Media Attributions
- U02-087 milk infographic © Brunning, Andy & Compound Interest is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-088 solvation shell Na © Berg, Steven P. Dr. is licensed under a Public Domain license
- U02-089 hydration shell NaCl © Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster is licensed under a CC BY (Attribution) license
- U02-090 Purple Cation Hydration Shell © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-091 Sulfate Ion Hydration Shell © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-092 Sugar in Water © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-094 and U02-095 and U02-096 avogadros number © Libretexts adapted by Jordan West is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- U02-097 strength of solution © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. is licensed under a CC BY (Attribution) license
- U02-098 Osmotic_pressure_on_blood_cells_diagram.svg © LadyofHats is licensed under a Public Domain license

