Proteins
Objective 3.3
3.3.1 Classify a protein as a polymer of amino acids.
3.3.2 Define the primary sequence of a protein.
3.3.3 Recognize α helices and β pleated sheet structures.
3.3.4 State how amino acid –R groups form shapes which are essential for most protein functions.
Because a protein is made from many similar small molecules (amino acids) joined by peptide bonds, it is an example of a polymer.
Proteins are amino acid polymers. Amino acids are the monomeric units that form protein polymers.
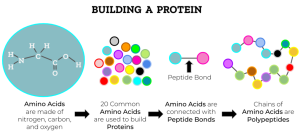
The primary sequence of a protein is the order in which amino acids appear in the protein. These are usually numbered in scientific papers and databases, so that the first amino acid (with a free amino end) is usually Met1, followed by the three-letter abbreviation and a sequential number.
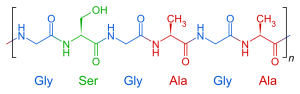
The primary sequence of a protein is held together by covalent bonds (peptide bonds) and is generally not easy to break. However, in the stomach and intestines, specialized enzymes break apart peptide bonds and for that reason are called peptidases. These digestive enzymes break proteins in the diet into one, two, or three amino acids (amino acids, dipeptides, or tripeptides) and those small amino acids or peptides can be absorbed through the intestinal wall.
Proteins are made by a microscopic “machine” or organelle called a ribosome. We will study the function of ribosomes in Unit 6. Ribosomes “stitch” together amino acids in a specific sequence, joining them with peptide bonds. DNA molecules are made into messenger RNA (mRNA) and the sequence of amino acids encoded in the mRNA is then turned into a primary sequence of protein by the ribosome.
Protein names generally end in the letters –in. That makes it easy to spot protein names you might be unfamiliar with: hemoglobin, myoglobin, actin, myosin, tubulin.
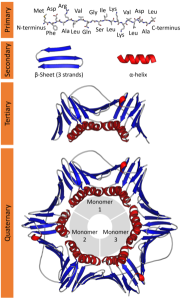
As mentioned earlier, the special properties of the –R groups of amino acids lead to the wide variety of structures seen in proteins. Proteins are folded into complex patterns. We recognize four levels of structure: primary, secondary, tertiary, and quaternary.
We used to require that students learn examples of each of these, but not any more! Now, in this edition of the textbook, we will focus on the specialized structures formed by proteins, including “tunnels”, “gates”, “tongs”, and “pockets”.
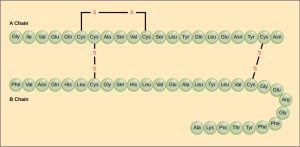
Proteins accomplish all their roles by having a remarkable range of structures. Protein structure starts with a primary sequence or primary structure. Which is the order in which amino acids are strung together with peptide bonds. For example, part of the insulin molecule (the “A chain”) in humans is:
Gly–Ile–Val–Glu–Gln–Cys–Cys–Thr–Ser–Ile–Cys–Ser–Leu–Tyr–Gln–Leu–Glu–Asn–Tyr–Cys–Asn
The primary structure may be twisted or folded into a secondary structure.
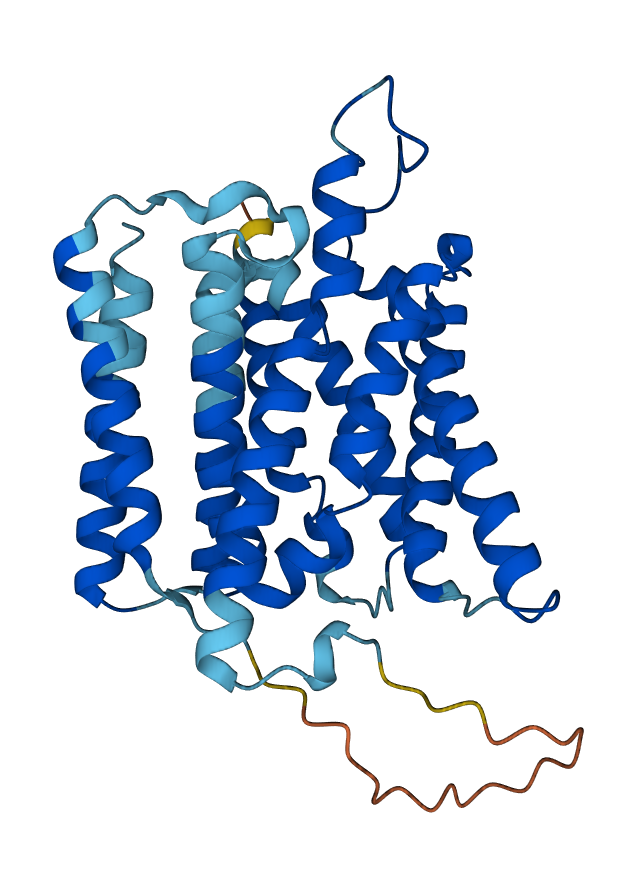
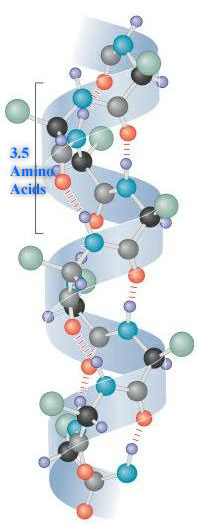 The α-helix is a common secondary structure and is found in many human proteins. Many proteins that span the membrane are made up of α-helices with the –R groups of hydrophobic (non-polar) amino acids associating with the hydrophobic lipids of the cell membrane and the –R groups of hydrophilic (polar) amino acids forming the pore.
The α-helix is a common secondary structure and is found in many human proteins. Many proteins that span the membrane are made up of α-helices with the –R groups of hydrophobic (non-polar) amino acids associating with the hydrophobic lipids of the cell membrane and the –R groups of hydrophilic (polar) amino acids forming the pore.
Hair is an α-helical structure formed from the protein keratin. (Notice the –in protein ending we mentioned earlier.) Each keratin molecule is a sequence of amino acids (primary sequence) twisted into an α-helix.
To make proteins, the ribosome “spinning wheel” which makes proteins from amino acid must turn incredibly fast. As you read this, the hair follicles on your head are making the α helices of keratin at the rate of 26 amino acids per second or 7 turns of the α helix per second. A single turn is 0.5 nm and there are 2.6 x 106 seconds per month. This works out to 1.25 cm per month or 15 cm per year. (We used Dimensional Analysis to figure this out. Just sayin’.)
Another common type of secondary structure is the β-pleated sheet. Notice in this diagram how hydrogen bonding holds two primary polypeptide sequences together in a β-pleated sheet.
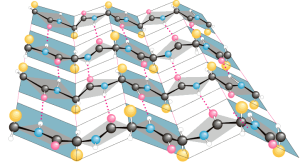
In many figures, α-helical regions are shown as spirals or by a straight tube. β-pleated sheets are usually shown as arrows (β-pleated sheets have a direction, which we won’t worry about now) or as flat ribbons. The regions of primary, secondary, tertiary (and sometimes quaternary) structure give a protein its special properties.
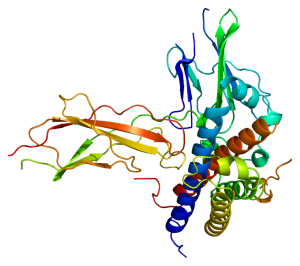
For example, the growth hormone molecule shown here, which we will study in Unit 14, fits into a pocket in another protein, the growth hormone receptor. It is the shape of the hormone and its receptor which allow the specific interactions to take place that result in growth of bone tissue.
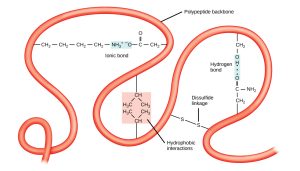
Protein structure is maintained by a variety of different kinds of interactions, most of which were covered in Unit 2.
- Ionic bonds are formed when positive and negative charges (i.e. acidic and basic amino acids) attract each other.
- Hydrophobic interactions occur where non-polar groups snuggle up to each other, excluding water.
- Disulfide bridges are formed between cysteines. Cysteine is the only amino acid with an –SH group.
- Hydrogen bonds are formed when a pair of O, N or S atoms share a hydrogen atom between them.
Insulin
In some proteins, two or more subunits combine together.
For example, three A chains and three B chains of insulin make up a complete signaling molecule.
These figures will help you review the essential features you need to know about protein structure, using representations of the signaling molecule insulin as our example in each case.
 |
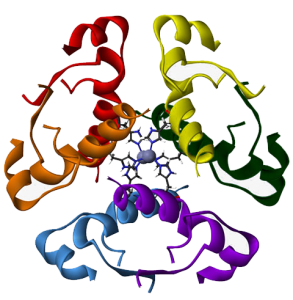 |
| linked insulin A and B chains | insulin hexamer |
Shapes which are commonly found in a variety of proteins are called protein motifs. For example, α-helices are often arranged so that the non-polar amino acid –R groups stick out of the spiral helix. This allows the hydrophobic –R groups to interact with the hydrophobic phospholipid tails (Objective Lipids & the Cell Membrane) and anchors the protein in the cell membrane. This anchoring is essential for the function of membrane-bound receptors and “signal flags” that identify specific cell types to other cells.
Another common motif is the helix-turn-helix motif. This, and a related motif called the zinc finger, are used to bind to specific DNA sequences and will sit in the grooves of a DNA double helix. This motif acts like tongs to grab DNA and turn specific genes “on” or “off” (Unit 6 Objective 3). Understanding the regulation of gene expression by these transcription factors is transcription factors is essential for many different fields of medicine, including embryological development, immunology, and cancer.
 |
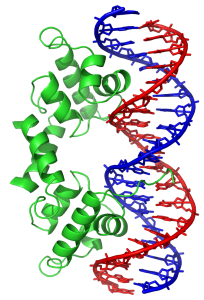 |
| Transmembrane Proteins: α-helical regions | DNA-binding Protein: helix-turn-helix motif |
The structures formed by proteins can be elaborate, functional, and beautiful. An example is the β-barrel motif, which uses parallel strips of β-pleated sheets to form a pore or barrel in the cell membrane. For example, the structure shown here forms a sucrose pore in some bacterial cell walls.
This motif is typical of a channel, a sort of tunnel that lets larger molecules or ions pass through the cell membrane. These channels can be gated, with a door that opens and closes, or ungated, like the sucrose pore shown here.
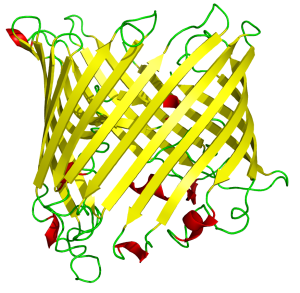 |
| Sucrose-specific Porin: β-barrel motif |
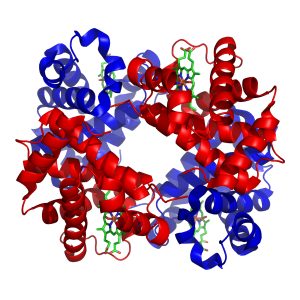
A common feature of many different types of proteins is the formation of a pocket lined by the –R groups of amino acids needed to hold onto a substance (binding sites), or carry out a chemical reaction (catalytic sites). We’ve seen hemoglobin molecules before. Hemoglobin is a protein that carries oxygen between the lungs and the cells of the body (Unit 17). Myoglobin is a similar protein which carries oxygen in muscle cells (Unit 10). Both hemoglobin and myoglobin use a Fe2+ ion to bind O2 molecules. The iron is embedded in a molecular structure called heme. The heme in turn sits in a pocket which uses specific amino acid –R groups to hold the heme tightly. Finally, the surrounding protein can change shape slightly to make the iron-heme group hold oxygen more or less tightly.
 |
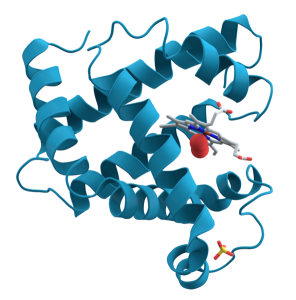 |
| Hemoglobin: iron-containing heme group | Myoglobin: iron-containing heme group |
Another class of pockets is found in the proteins of the immune system, immunoglobulins. (Note the –in protein name ending.) Immunoglobulins (we will see in Unit 15) bind to an incredible array of molecules, including lipids, sugars, and proteins which either act as toxins in the blood or those molecules found on the surface of invading bacteria, fungi, viruses, or parasites. In this context, immunoglobulins are called antibodies, like the antibodies to SARS-CoV-2 coat protein that your body makes when you’re vaccinated. The immune system makes over 4 billion kinds of pockets by shuffling different genes, and then eliminates those pockets that bind to molecules found within the human body. The immunoglobulins which are left over have pockets that you may or may not ever use, but with hundreds of millions of different shapes which can (we hope) bind to anything we might encounter in a lifetime.
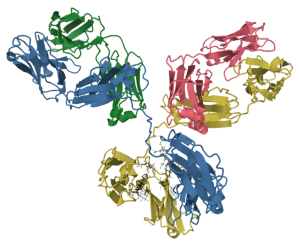 |
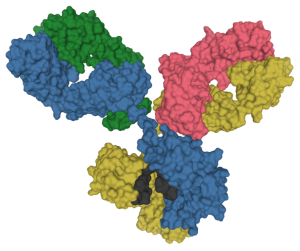 |
Media Attributions
- U03-034 building a protein © SadiesBurrow is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-035 primary protein structure © Sponk is licensed under a Public Domain license
- U03-037 protein structure alpha helix © National Institutes of Health is licensed under a Public Domain license
- U03-038 protein structure beta pleated sheet © Preston Manor School + JFL is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-043 Protein_structure_(full) © Shafee, Thomas is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-039 protein structure growth hormone © Emw is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-040 protein structure sources © Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster is licensed under a CC BY (Attribution) license
- U03-041 protein structure tertiary © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U03-044 insulin-hexamer-3d-ribbons-human-insulin-protein © Benjah-bmm27 is licensed under a Public Domain license
- U03-045 Human_Transmembrane_144_Protein_Tertiary_Structure © Walko031 is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-046 Lambda_repressor_1LMB © Zephyris is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-047 Sucrose_porin_1a0s © Opabinia regalis is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-048 hemoglobin © Zephyris is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-049 protein structure Myoglobin2 © AzaToth is licensed under a Public Domain license
- U03-050a Antibody IgG1 structure © Tokenzero is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-050b Antibody IgG1 structure © Tokenzero is licensed under a CC BY-SA (Attribution ShareAlike) license

