Photons
Objective 2.2
2.2.1 State the correct order of energy (wavelength) in the electromagnetic spectrum: visible light, ultraviolet, X-rays, gamma rays.
2.2.2 Describe how the wavelength and energy of photons are related.
Photons are particles of light. Photons also behave as waves. It’s another weirdness of quantum physics that photons are both a particle and a wave. This seems weird at first, but we know of a lot of things that are a superposition of two states: you might be both an American and a college student. Just like you can be two things at once, and what you are depends on what questions we ask, so photons behave as particles in some experiments and waves in others.
As particles, they carry a certain energy. For example, a bullet is a pretty small piece of metal, but it moves at a very high speed so it carries a lot of energy and can do much damage.
In classical physics,
Einstein’s Theory of Relativity says photons have zero mass but a measurable momentum. That’s strange, because anything multiplied by zero should be zero, so if mass = 0, then mass × velocity should equal zero.
![]()
Einstein also said the velocity of photons (“speed of light”) is a constant: 3 × 109 m/sec.
Photons arise from an excess amount of energy that results through at least three processes that involve rearrangement of subatomic particles:
- nuclear rearrangement
- nuclear fission (splitting)
- electron rearrangement
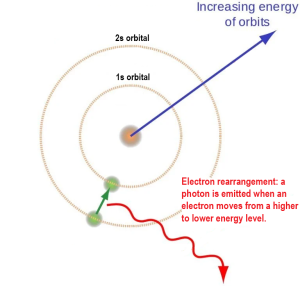
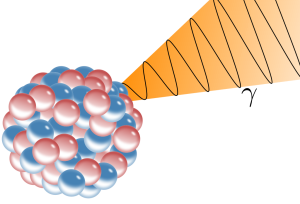
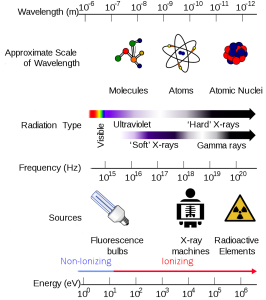
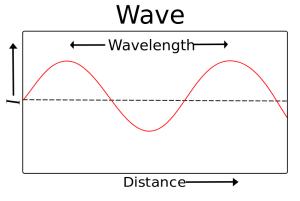
Visible light, ultraviolet light, X-rays and gamma rays are all “made” of photons but with different momentum (energy) and different wavelengths. A wavelength is the distance between the crest of waves. The height (amplitude) of the wave is the intensity of the signal (e.g a bright light vs a dim light).
The wavelength is what makes a difference between different forms of electromagnetic radiation. The wavelength is also related to the energy carried by the wave-particle. High energy photons have short wavelengths; low energy photons have long wavelengths. (Waves crashing on the beach several times a second have a lot more energy than a few waves a minute.)
Visible light has a wavelength in the range of about 400 to 700 nm (0.4 to 0.7 μm). The longest of these, 700 nm, is also the lowest energy (red). The shortest of these, 400 nm, is also the highest energy (violet). From longest to shortest wavelength (lowest to highest energy), the colors are red, orange, yellow, green, blue, [indigo] and violet. The mnemonic for this sequence is ROY G. BiV who is said to be the “discoverer” of light.
In the visible spectrum, the color violet has the highest energy and shortest wavelength. Beyond violet in the visible light spectrum violet is the invisible spectrum, starting with ultraviolet, and then X-rays and gamma rays.
Ultraviolet (UV) has enough energy to cause first-degree burns to tissue (a sunburn) or to cause scar tissue to form in the tissues of the eye (cornea or lens).
X-rays are photons with a high enough energy to penetrate living tissue, so they are used for medical diagnostics. When X-rays strike film, they produce a black color, so dense tissue like bone (which stops X-rays) appears white under X-ray.
Gamma rays are photons with enough energy to penetrate tissue, but they can also damage living tissue. For that reason, they are used to destroy tissue that cannot be reached by surgical means.
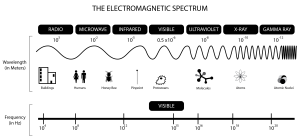
Notice in this image that the shorter the wavelengths are, the higher the energy of the electromagnetic wave. On the low end of the energy spectrum are radio waves with the crest of the wavelengths far apart. On the high end of the spectrum are gamma rays. Look how close together the wavelengths are. The closer the wavelengths, the higher the energy.
Media Attributions
- U02-008b momentum graphic © Bizell, Lizz is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-009 Edited Bohr photon image © Enoch Lau is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-010 Gamma_Decay.svg © Inductiveload is licensed under a Public Domain license
- U02-011 electromagnetic spectrum hutchins mod © Dinksbumf, Inductiveload, and NASA is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-012 800px-Wavelength.svg © Ysangkok is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-013 EM_spectrum © Urie, Jonathan S. is licensed under a CC BY-SA (Attribution ShareAlike) license

