Passive Transport Across Membranes
Objective 4.5
4.5.1 Describe passive transport processes and the effects of osmotic pressure on cells to include simple diffusion, facilitated diffusion, osmosis, tonicity, and filtration.
Diffusion videos
Khan Academy: Diffusion and Osmosis
Remember, the fluids of our body contain water and stuff (solutes). For cellular processes to take place, substances must be transported across the cell membrane, from the intracellular compartment to the extracellular and visa versa. There are a number of transport processes, and they can be classified as passive or active, depending on whether or not they require the use of cellular energy. Energy is necessary if a substance is transported “uphill” or “against” its concentration and/or electrical gradient. You may ask, “What is a gradient?” Think of it as a difference (magnitude). If there is a higher extracellular concentration of Na+, there is a concentration gradient, and because they are ions, an electrical difference (gradient) exists as well. If the cell wants to transport more Na+ to the extracellular side, its going to require energy to do it. Its like pumping water uphill. We will focus on specific active transport processes in an upcoming objective.
On the other hand, if you pour water downhill, it doesn’t require any energy. Passive transport processes function this way. They don’t require energy because substances (water or solutes) are transported “downhill” or “with” their concentration or electrical gradients. Instead of pumping water, this is like opening the spillway of a dam and just letting the water flow out.
The passive transport processes we will study are:
- diffusion,
- facilitated diffusion,
- osmosis, and
- Filtration
They are all the same in that we will move substances with their gradients, and we will not require energy to do it. So what makes them different?
Ask yourself these questions. Am I moving solute or solvent? And, are you requiring the assistance of a protein (channel or transporter) or filtration barrier to move the substance?
The laws of diffusion were laid down by a German chemist, Adolph Fick, in 1855. For that reason, they are called Fick’s Laws. In this course, we don’t need to know Fick’s Laws, but it’s fun to say his name. We’ll study a simplified version of Fick’s Laws. Fick’s First Law has several parts, but we’ve already mentioned a fragment of the First Law: molecules always diffuse from an area of high concentration (as in the unstopping of a perfume bottle) to an area of low concentration (the air in the room where the bottle is opened).
Diffusion is an example of increasing entropy. If we put a bunch of socks in a drawer, and then wait, over time, the socks will distribute randomly through the drawer.They each go from areas of high concentration to low concentration. Molecules do the same. If we want to put socks back in order, we have to input energy (taking time to sort the socks) and we might even include an energy barrier (drawer dividers) to keep the socks sorted.
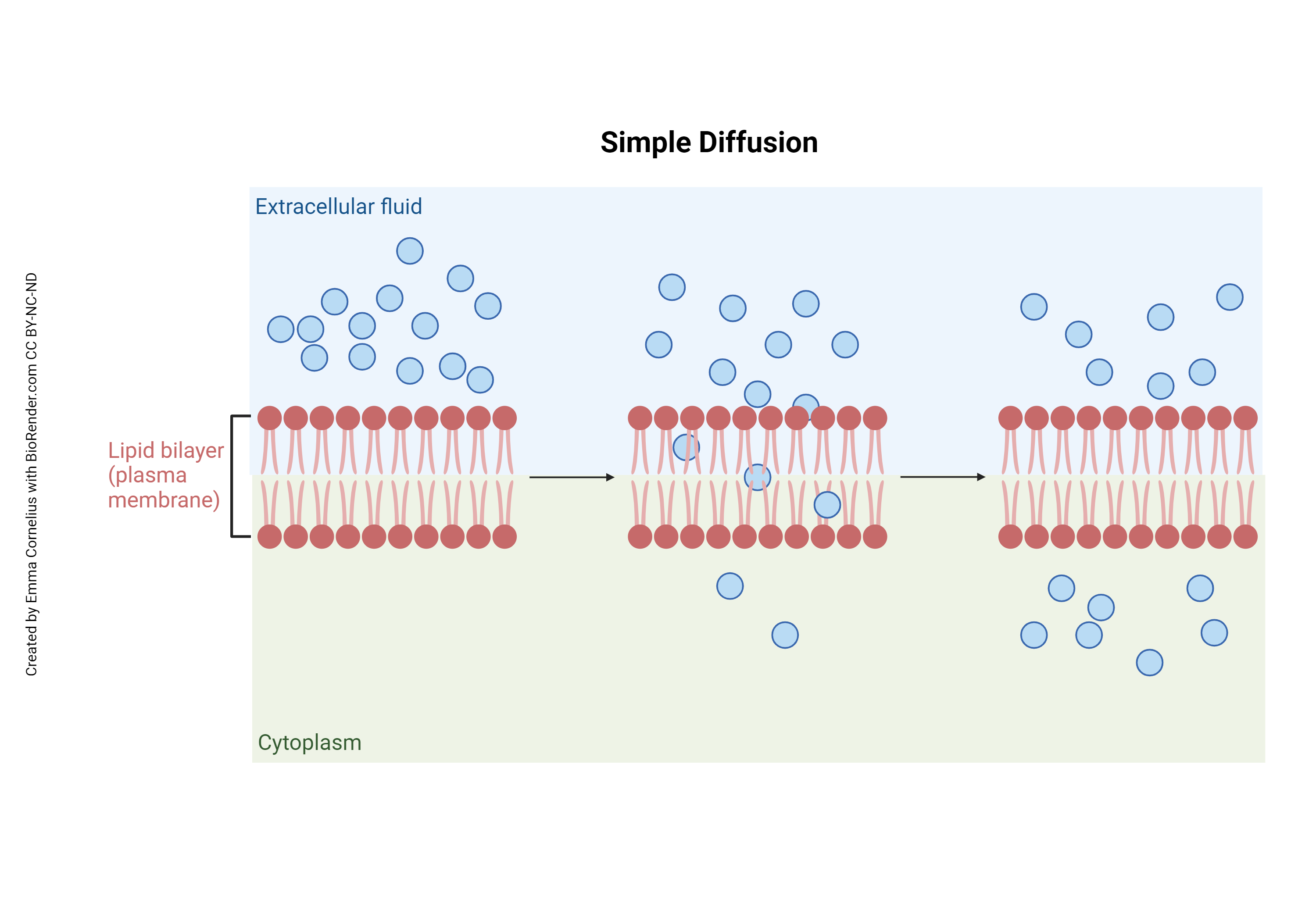
Simple Diffusion
In this picture, notice in the first frame all of the small, uncharged molecules are in the extracellular space. They begin to diffuse from a high concentration to a low concentration as time progresses. Eventually these molecules will diffuse until they are equal across the cell membrane.
 We see many examples of simple diffusion in our everyday life. If we add a drop of food coloring to water, we expect that the color would spread throughout the water until the water becomes the color of the dye. The molecules in the food coloring are simply diffusing from an area of high concentration to low concentration.
We see many examples of simple diffusion in our everyday life. If we add a drop of food coloring to water, we expect that the color would spread throughout the water until the water becomes the color of the dye. The molecules in the food coloring are simply diffusing from an area of high concentration to low concentration.

For example, if an aroma diffuser, or essential oil diffuser, were first turned on, the aromatic smell would be strong close to the diffuser. As these molecules diffuse from high to low in the air, the smell of the aroma also diffuses. We can guess, from perfume in air or dye in water, that molecules in a liquid or gas are free to move around. Molecules in a solid move, but with much less freedom, so that if we put a drop of food dye on ice we get a much different result than if we put a drop of food dye in liquid water. This random motion of small particles or even molecules in a liquid or gas is called Brownian motion.
If we think of molecules as billiard balls, and temperature as the speed of the billiard balls, then it makes sense that a higher temperature results in more diffusion (more collisions, and more motion). Similarly, small billiard balls move more readily than big, basketball-sized ones. Taking all this together, molecules in a liquid or gaseous medium tend to spread themselves out evenly throughout the volume of the container. This property is called diffusion.
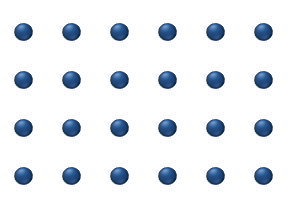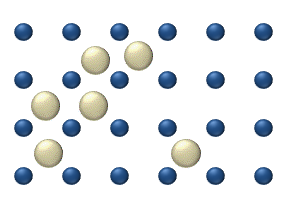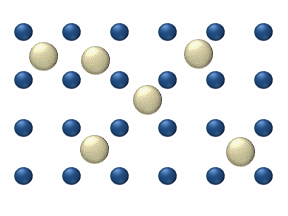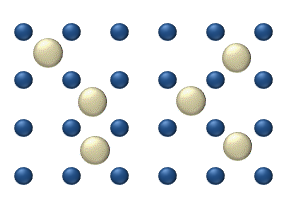
Small, neutrally-charged, lipid-soluble substances can move across the cell membrane without help. For other solutes, some sort of escort is necessary, a channel or transport protein. That is what distinguishes simple diffusion from facilitated diffusion.
Facilitated Diffusion
We learned earlier that charged ions or molecules cannot easily pass through the cell membrane. They require a bridge. In the cell, this bridge is a protein. If help is required to pass the cell membrane, but molecules are still moving from high to low concentration, they are facilitated in their journey. This is still passive diffusion because no energy is required.
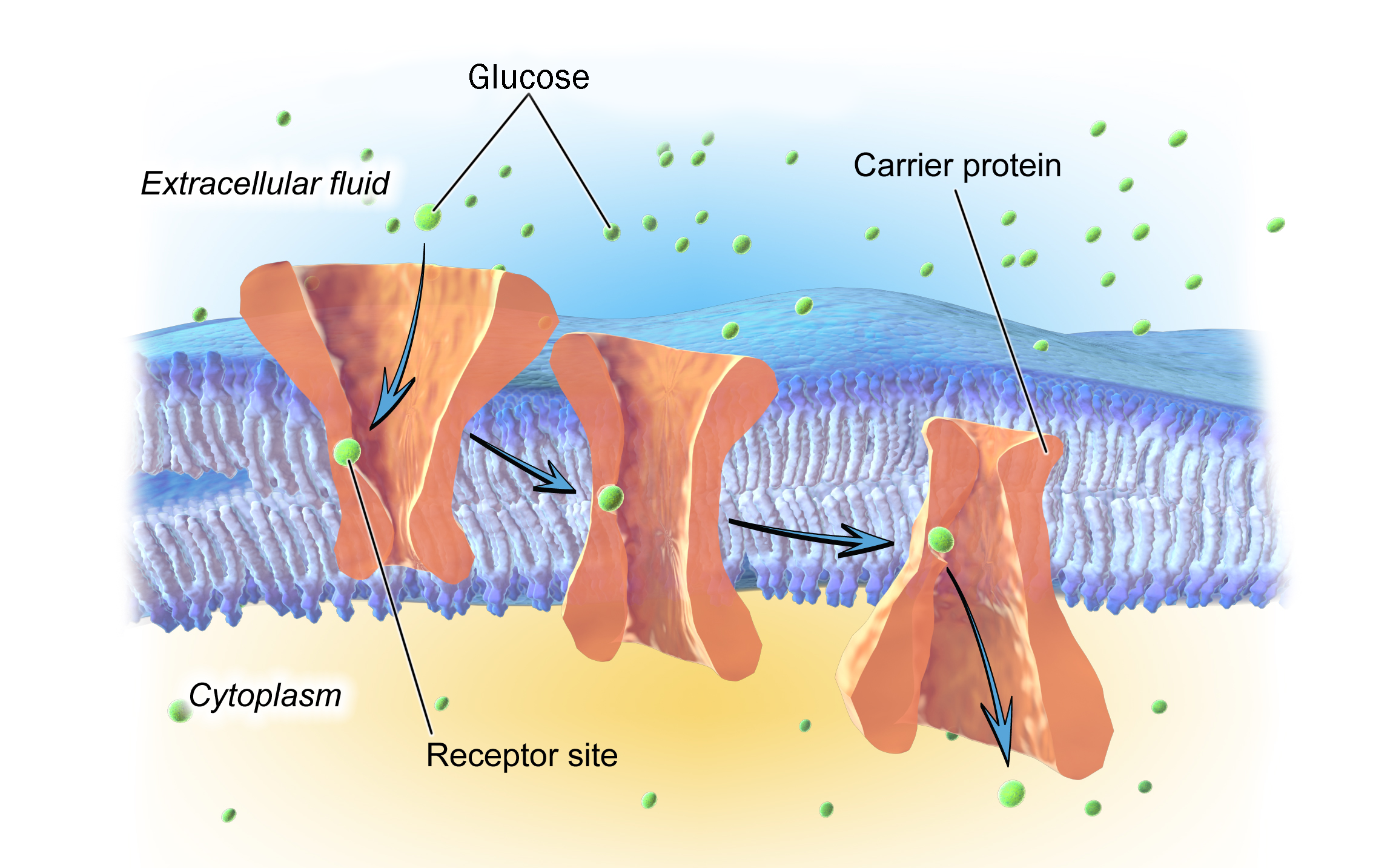
Some specific examples of facilitated diffusion will help us to understand the concept. We’ll take potassium (K+) first. We learned in the chemistry module that KCl, and most other K+ containing compounds, dissolve in water to form ions (K+ and an anion). We learned in this module, that ions such as K+ don’t pass through the lipid bilayer without assistance so it can’t move by simple diffusion. Finally, we need to know that K+ is at much higher concentration inside the cell than outside. If allowed to diffuse, then, K+ will move from where it is at higher concentration to where it is at lower concentration (that is, from inside the cell to outside).
In the cell membrane, there are channel proteins with pores that only allow K+ ions to pass. These channel proteins are gated. When the gate is open, K+ can flow out of the cell. When the gate is closed, K+ cannot pass and is trapped inside the cell.
Another example of facilitated diffusion is glucose. Glucose is not dissolved as an ion; it is a complete true molecule in solution. It is water-soluble (hydrophilic) and will not pass through the lipid bilayer without assistance. It is at higher concentration outside than inside (if escorted, it will diffuse from outside the cell to inside). The cell membrane has glucose transporters that move glucose down its concentration gradient, from outside to inside the cell.
Osmosis
Now that we understand the two types of diffusion, and how cells use diffusion, let’s add a further twist. We need to understand the process of osmosis. Keep in mind with diffusion and facilitated diffusion we moved solutes. Osmosis is a term that describes the diffusion of solvents across a semi-permeable membrane. For us, there’s only one solvent, water. The semi-permeable membrane is the cell membrane (“semi-permeable” means it allows some things across but not others, a property of cell membranes we’ve already explored).
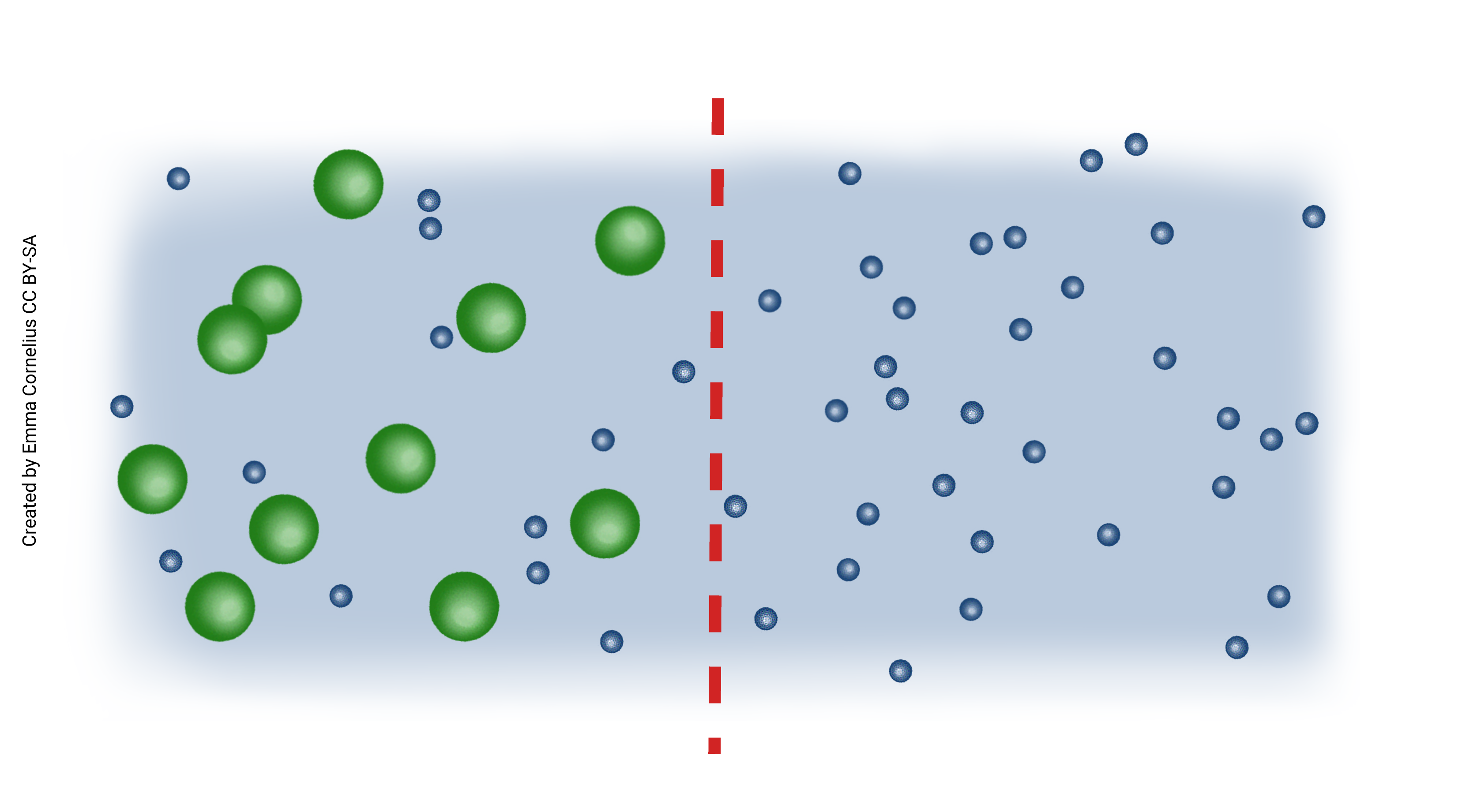
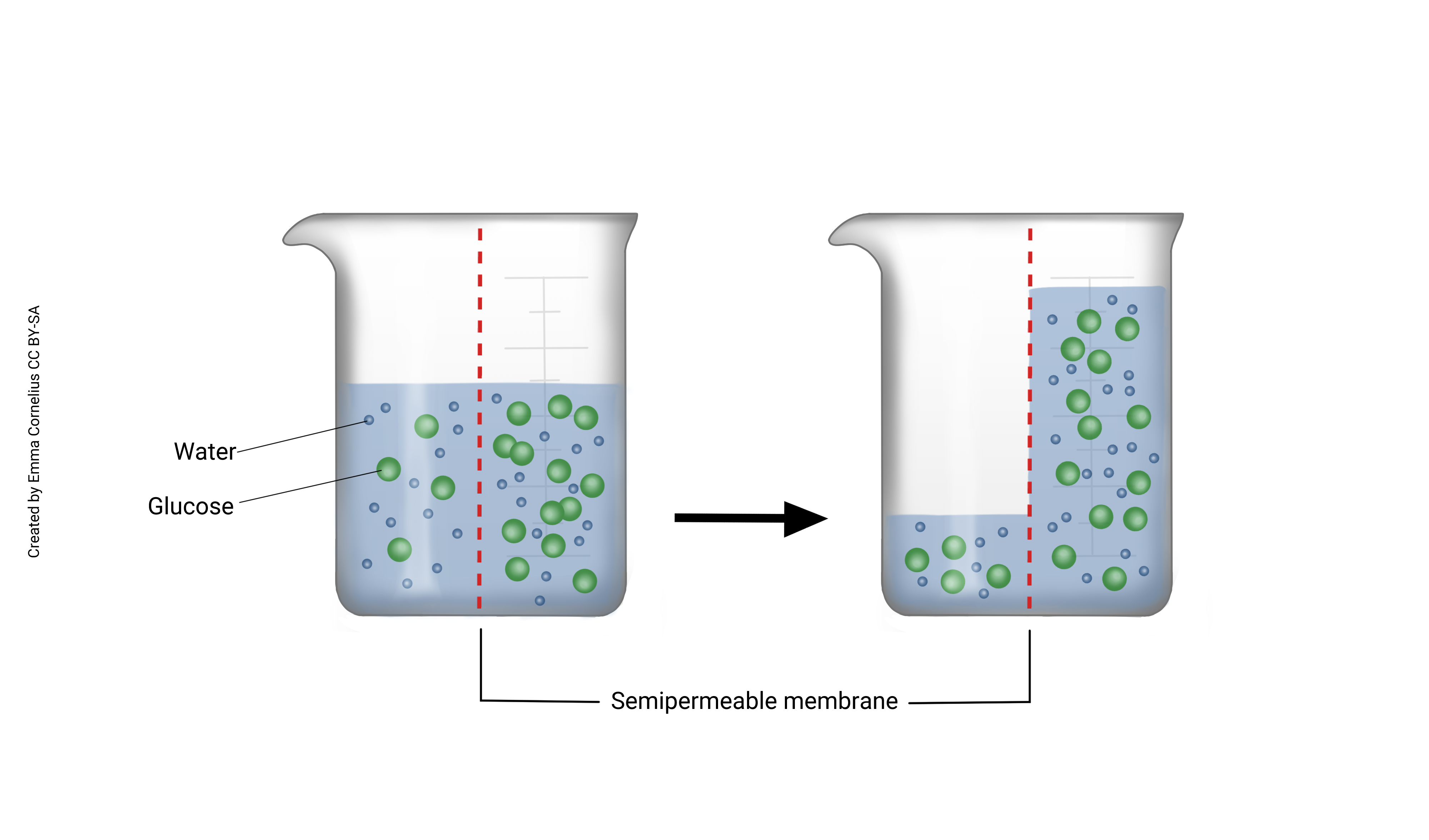
When we combine diffusion of water with a semi-permeable membrane, we get osmosis. Imagine, for example, a two-chambered vessel divided by a semi-permeable membrane that allows passage of water but not sugar (see figure left). Sugar molecules are depicted as green balls and water as the smaller blue balls. The sugar molecules are too big to pass through the semi-permeable membrane but the water molecules can freely diffuse. Notice that there are more water molecules on the right, where there is less solute (sugar). Water is diffusing from a high concentration to low (passively) across the semi-permeable membrane. You can also think of this as water moving to dilute the higher concentration.
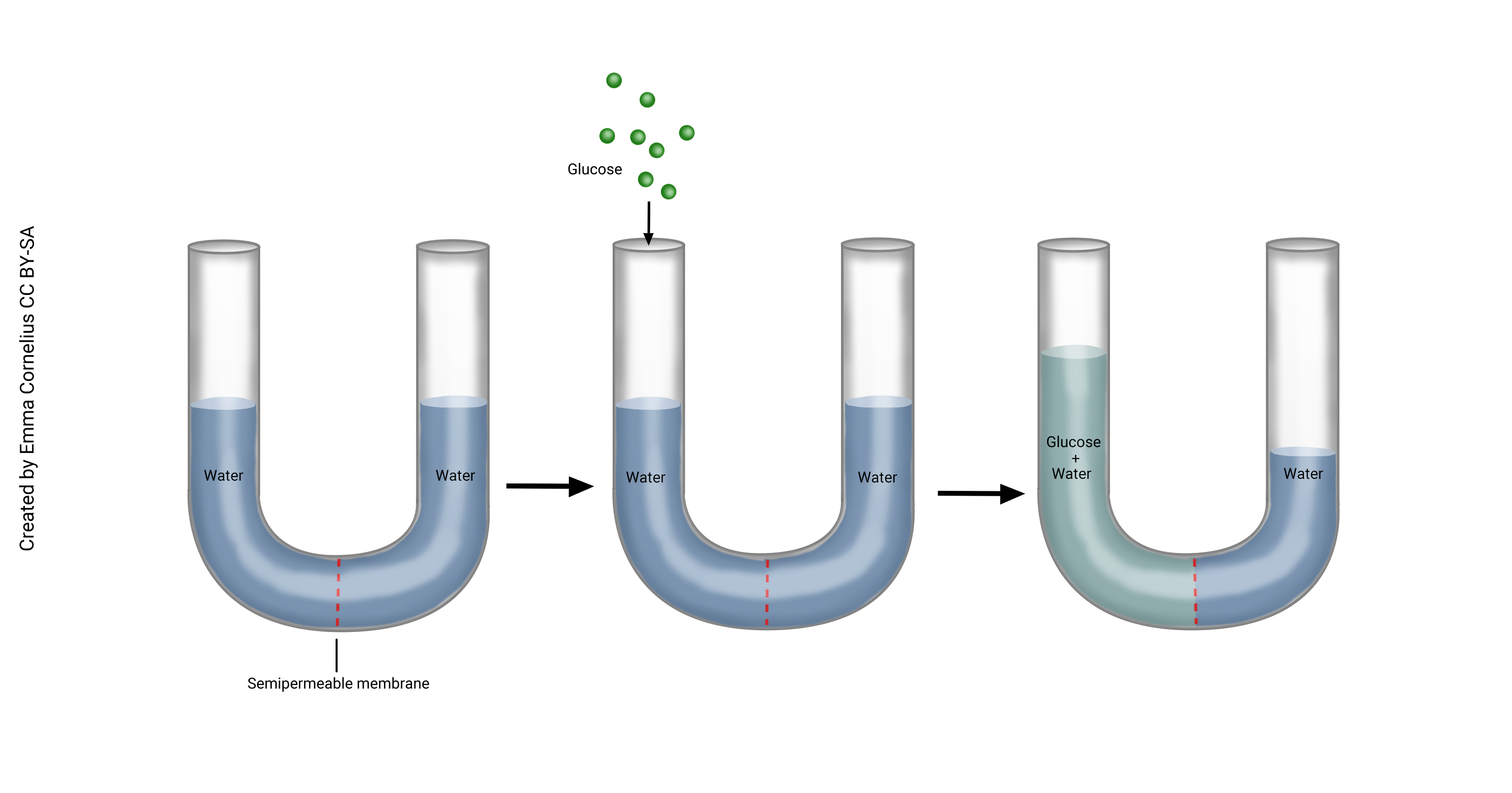
Below, this U-shaped tube is divided by a semi-permeable membrane. In the diagram in the left frame, water has diffused to be equal across the membrane. Now imagine adding glucose to the left side of the beaker. Glucose is a large molecule and cannot diffuse across the membrane, but water still can. Because the glucose is taking up room, water is now at a higher concentration to the right of the membrane. Notice in the frame on the right, water has diffused from right to left (high to low concentration) and is making the fluid level on the left of the beaker higher. This is osmosis. We can think about this another way which sometimes makes it easier when talking about the movement of fluids in the body. When glucose is added to the beaker on the left, this solution is now a higher concentration than the water on the right. Water moves to dilute the higher concentration.
Now imagine that we place a cork on the left side of the beaker, after adding the glucose. Although water still wants to move from the right side of the beaker to the left, we’ve prevented this by not allowing the water on the left side to rise. Osmotic pressure is the pressure needed to prevent the water from passing through the semi-permeable membrane.
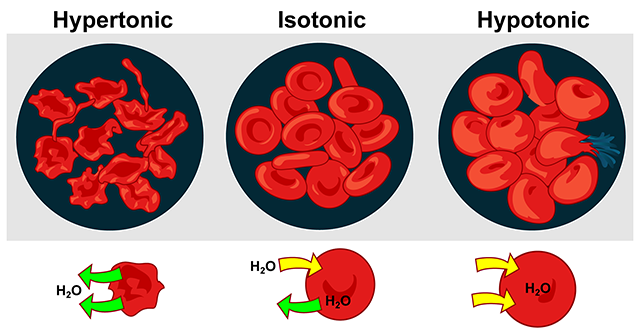
Changing Tonicity on Cells
We use tonicity to compare the osmotic pressure of two solutions separated by a semipermeable membrane. In the context of our physiological processes, the two solutions are the intracellular fluid and the extracellular fluid, separated by the cell membrane (semipermeable).
An understanding of diffusion and osmosis are fundamental for an understanding of tonicity. Tonicity is just another way of describing the effect of osmotic pressure on cells.
In isotonic (Greek isos, “equal”) solutions, the osmotic pressure of the extracellular solution is the same as the osmotic pressure inside the cell, and because the pressures are equal, the net movement of water is “zero”. Thus the cell remains the same shape and size. For example, nine grams of NaCl in a liter of water (0.9% NaCl solution), is isotonic to blood plasma. An example frequently used in medicine is Ringer’s solution, an intravenous (IV) solution used for fluid replacement.
In hypotonic solutions, the concentration of solutes is lower outside the cell than inside. Water moves into the cell, and causes it to swell. In distilled water (a very hypotonic solution), red blood cells will swell until they burst, or lyse. Using water as intravenous fluid replacement in patients would be very stupid for this reason.
In hypertonic solutions, the concentration of solutes outside the cell is higher than inside. As water moves down its concentration gradient (inside → outside), the cell shrinks. When red blood cells shrink in a hypertonic solution, we say they crenate: they take on a spiny shape, which is not good if the RBC needs to slide easily through blood vessels.
Filtration
Cells depend on a variety of mechanisms to move things from inside to outside the cell, or to move them from outside to inside the cell. We have already studied three of these in detail: simple diffusion, facilitated diffusion, and osmosis. Filtration is another type of passive transport. In filtration, solutes and colloids pass through a filtration barrier based on size.
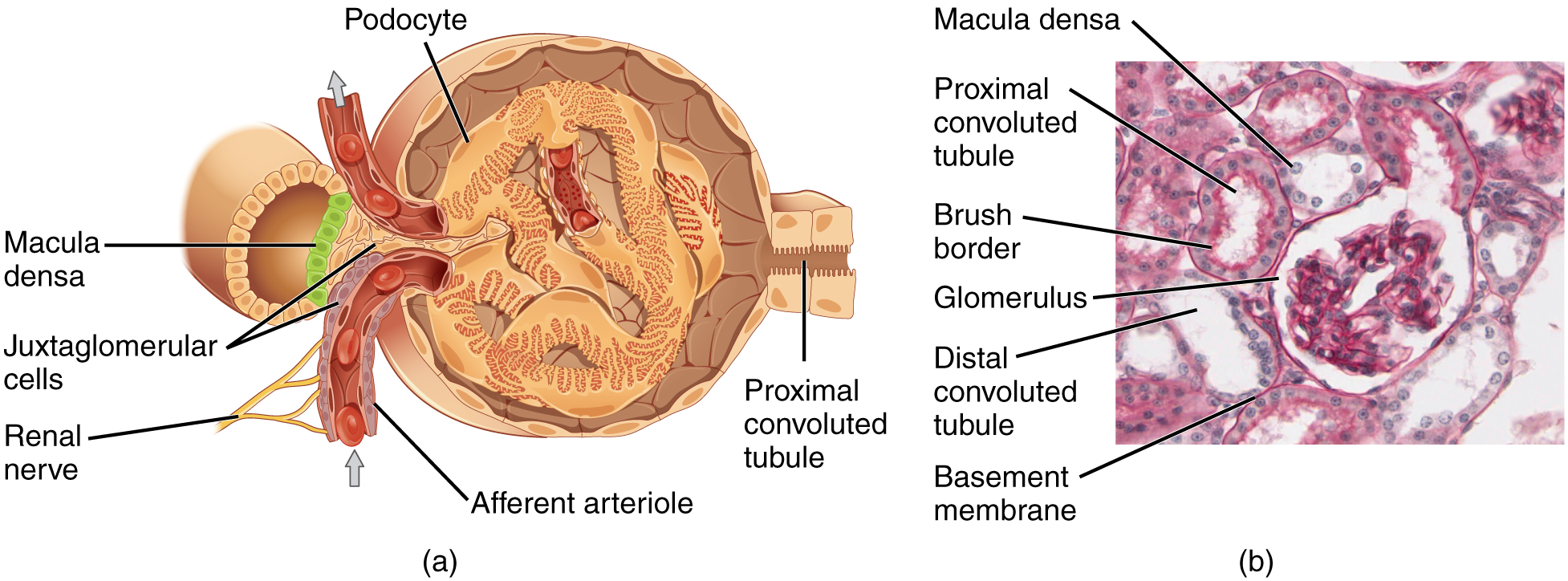
Substances are filtered in the part of the kidney known as the glomerulus, substances less than 10 kDa (10,000 Da) will pass through the biological filter system. Sugar and amino acid monomers are about 100 Da in size, on average. Therefore, the biological filter in the kidney passes all monomers, and also sugar and protein polymers of smaller size. For example, albumin, the most abundant protein in the blood, is 68 kDa in size. Very little albumin passes through the kidney’s filter system if it is healthy.
This picture shows the glomerulus, the filtering unit of the kidney. We’ll learn more about this filter in the renal unit.
Media Attributions
- U04-030 Simple diffusion © Cornelius, Emma is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U04-031 flickr zacharmstrong CC BY-NC-ND © Armstrong, Zachary is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U04-033 Chemical_surface_diffusion_slow © Runningamok19 is licensed under a CC BY (Attribution) license
- U04-034 Blausen_0394_Facilitated_Diffusion hutchins mod © Blausen, Bruce is licensed under a CC BY (Attribution) license
- U04-035 Osmosis 1 © Cornelius, Emma is licensed under a CC BY-SA (Attribution ShareAlike) license
- U04-036 Osmosis 2 © Cornelius, Emma is licensed under a CC BY-SA (Attribution ShareAlike) license
- U04-037 Osmosis 3 © Cornelius, Emma is licensed under a CC BY-SA (Attribution ShareAlike) license
- U04-038 Osmotic_pressure_on_blood_cells_diagram LadyofHats wikipedia public domain © LadyofHats is licensed under a Public Domain license
- U04-039 Glomerular filtration © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license

