Parts of the Atom
Objective 2.1
2.1.1 Name the elementary particles that make up atoms: neutrons, protons, and electrons.
2.1.2 Describe the charge, mass and relative location of neutrons, protons, and electrons.
 All matter is made up of atoms. An atom is a particle that contains all the characteristics of an element. That is, one atom acts chemically like two hundred, or two thousand, or two billion atoms.
All matter is made up of atoms. An atom is a particle that contains all the characteristics of an element. That is, one atom acts chemically like two hundred, or two thousand, or two billion atoms.
An atom used to be an imaginary thing. Now, with certain kinds of imaging, scientists can actually “see” atoms directly.
Atoms make up the smallest unit of matter that retain the properties of their associated element. A pure element (like the carbon in a diamond, or helium gas in a balloon) consists of atoms, all of which have identical chemical properties.
The most common elements in the human body (and the symbols which represent them) are:
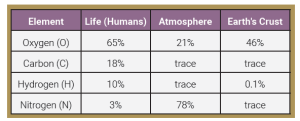
- oxygen (O)
- carbon (C)
- hydrogen (H)
- nitrogen (N)
The graph indicates approximate percentages of elements in living organisms (humans) compared to the nonliving world.
These are the elements that we will focus on in this course. Because elements are made by fusion reactions in the sun, the smaller, lighter elements are more common than the larger ones here on earth. We will list the elements that you need to know a bit later but we’ll start with the “top four”.
Properties of atoms that we will learn in this Unit come from both theoretical and experimental models of atoms and elements.
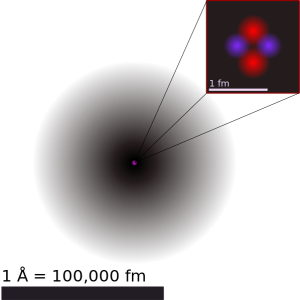 The diameter of an atom is about 10–10 m (0.1 nm, 0.0000001 mm, or 0.0000000001 m). An old name for this unit is an Ångstrom (Å). The diameter of a molecule is about 10 times that, or 1 nm. For this reason, when scientists build devices that work at the molecular (nanometer) scale, it’s called nanotechnology.
The diameter of an atom is about 10–10 m (0.1 nm, 0.0000001 mm, or 0.0000000001 m). An old name for this unit is an Ångstrom (Å). The diameter of a molecule is about 10 times that, or 1 nm. For this reason, when scientists build devices that work at the molecular (nanometer) scale, it’s called nanotechnology.
There are things smaller than atoms: subatomic particles. Physicists have lots of subatomic particles, but for our purposes, we will consider atoms to be made up of three elementary subatomic particles:
- neutrons
- protons
- Electrons
We’ll consider each of these in turn.
The nucleus is in the center of an atom and contains the massive (i.e. heavy) particles that make up atoms. The mass of atoms is measured by a unit that is (naturally enough) called the atomic mass unit (amu) or Daltons (Da).
A neutral atom of a particular element has an equal number of electrons and protons (as we’ll see later, this means the + and – charges cancel out, hence the term “neutral”) but the number of neutrons can vary. This variation changes the atom’s mass but not its chemical properties. Atoms which vary in the number of neutrons are called isotopes. We’ll take a closer look at isotopes in Objective 3.
Neutrons are located in the nucleus. They have no charge, but have a mass of 1.009 Da. For the purpose of this course, we round that to 1 Da.
The only time neutrons are apparent is when atoms break apart in a process called nuclear fission. Nuclear fission is the basis for nuclear weapons, nuclear reactors, and nuclear medicine.
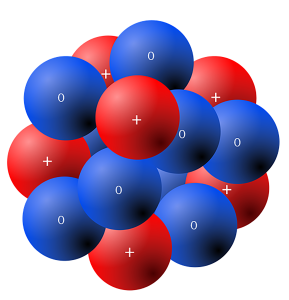 Protons are the other particles found in the nucleus. They carry a positive charge. Protons have a mass of 1.007 Da, but for our purposes, the mass is 1 Da.
Protons are the other particles found in the nucleus. They carry a positive charge. Protons have a mass of 1.007 Da, but for our purposes, the mass is 1 Da.
Like neutrons, they are sequestered in the nucleus and cannot participate in chemical reactions. Also like neutrons, the only time they are apparent is in nuclear fission.
We indicate the number of protons (called the atomic number) with a subscript number in front of the element symbol:
8O 6C 1H 7 N
 Electrons are small and light. (The weight is 9 × 10–28 g, or 0.0005 Da, 1/2000 the weight of a neutron or proton, but you do not have to learn it.) They have a negative charge. They are distant from the nucleus, so they are hanging out looking for things to do, like contributing to the chemical properties of the atom.
Electrons are small and light. (The weight is 9 × 10–28 g, or 0.0005 Da, 1/2000 the weight of a neutron or proton, but you do not have to learn it.) They have a negative charge. They are distant from the nucleus, so they are hanging out looking for things to do, like contributing to the chemical properties of the atom.
Electrons have been conceived as a number of different ideas: as particles, as waves, or as packets of negative charge (quanta) with a certain probability of existing in a specific place. That is, according to quantum theory, we can never really know where an electron is. Rather, we can just say where it’s more likely or less likely to be.
We will see later that atoms readily gain or lose electrons. A chemical reaction can alter the position (or probability function) of electrons, but a chemical reaction does not change anything about the nucleus—by definition, only a nuclear reaction can do that.
In a neutral atom, the number of packets of positive charge (protons) and the number of packets of negative charge (electrons) are exactly equal: all the positives cancel out all the negatives and the net charge of a neutral atom is zero.
But the number of electrons can change. When the number of electrons is no longer equal to the number of protons, we call this an ion. An ion has either more positive charges or more negative charges. A cation has more protons than electrons (i.e. an excess of positive charges). An anion has more electrons than protons (i.e. an excess of negative charges). We indicate the excess charges with a superscript after the element’s symbol:
8O
8 protons, 2 electrons: O6+
8 protons, 10 electrons: O2–
6C
6 protons, 2 electrons: C4+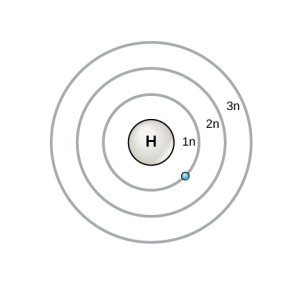
6 protons, 10 electrons: C4–
1H
1 proton, 0 electrons: H1+
(usually represented as H+)
1 proton, 2 electrons: H1–
(usually represented as H–)
7N
7 protons, 2 electrons: N5+
7 protons, 10 electrons: N3–
(Note that the number of protons is equal to the atomic number, as explained earlier. Also note that ions “like” to have zero, 2, or 10 electrons. This will be discussed later.)
Electrons sort themselves into energy levels. Electrons closer to the nucleus are at a lower energy level, while electrons further away from the nucleus are at a higher energy level.
In one model (called the Bohr or planetary model), these energy levels are represented by concentric circles.
Because quantum theory states that electrons are a probability distribution of the location of negative charges, a more complete understanding of electrons is to represent them as circular or pear-shaped probability clouds called orbitals. Orbitals can hold from one to seven pairs of electrons.
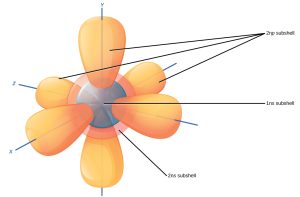
Electrons are paired by their spin (up or down); “up” electrons like to be next to “down” electrons.
Media Attributions
- U02-001 atom © Dooley, Kevin is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-002 common elements © Bizell, Lizz is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-003 Helium_atom_QM.svg © Yzmo is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-004 Nucleus_drawing © Marekich is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-005 nucleus drawing protons neutron charge labeled © Marekich adapted by West, Jordan is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-007 bohr model © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U02-008 subshells © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license

