Nucleic Acids
Objective 3.9
3.9.1 Define: nitrogenous base, nucleoside, nucleotide.
3.9.2 Recognize examples of nucleosides
Nucleic acids, as the name implies, are acidic molecules found in the nucleus of cells. The two forms of nucleic acid differ mostly by the sugar molecules in the backbone of the molecule: ribonucleic acid (RNA) has ribose in its backbone, while deoxyribonucleic acid (DNA) has deoxyribose in its backbone. Those are the two pentose sugars we studied in the carbohydrate section.
RNA and DNA are polymers of monomers known as nucleotides.
Nucleic acid polymers are huge molecules. There are 46 DNA molecules in each cell of the human body. The largest of these has 220 million nucleotide monomers, a molecular weight of 72 billion, and if stretched out would be 2 meters long. (That’s just one of 46 molecules found in each of your 10 trillion cells.)
Nitrogenous Bases
Nucleotides are monomers of nucleic acids. The core molecule in each is a nitrogenous base (adenine, cytosine, guanine, thymine or uracil, abbreviated A, C, G, T or U).
The bases are classified as pyrimidines or purines. Their structures are shown below.
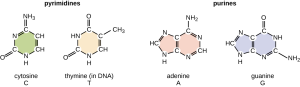
Adenine and guanine are larger, two-ring nitrogenous bases with five carbons and five nitrogen atoms each. These are called purines. (A mnemonic: the bigger molecule has the smaller name.)
Thymine, cytosine, and uracil are pyrimidines. These are single-ring nitrogenous bases. Thymine and uracil are identical except for the addition of a single methyl group to the thymine molecule. Thymine is found in DNA and uracil is found in RNA; they have similar functions in these two molecules, as we’ll see later.

Nucleosides
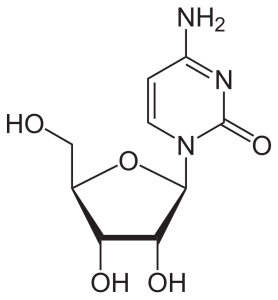
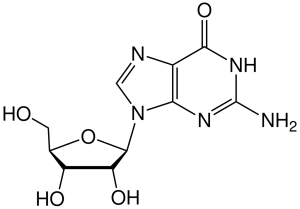
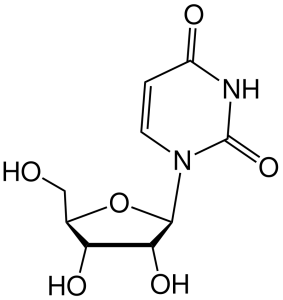
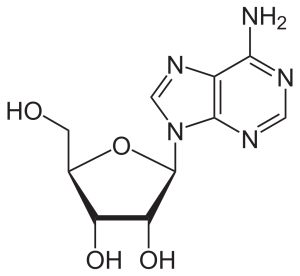
If a sugar (ribose or deoxyribose) is added to one of these nitrogenous bases, then a nucleoside results.
Nucleosides in RNA include:
- adenosine, cytidine, guanosine, and uridine
Nucleosides in DNA include:
- 2’-deoxyadenosine, 2’-deoxycytidine, 2’-deoxyguanosine, and 2’-deoxythymidine
 |
 |
 |
| cytidine | guanosine | uridine |
 |
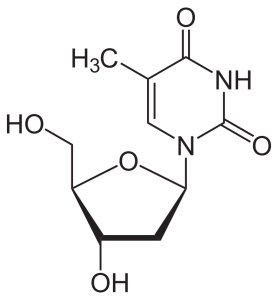 |
| adenosine | 2′-deoxythymidine |
Nucleotides
Nucleotides consist of a nucleoside plus a phosphate group. These phosphate groups link to a sugar in two places to form a chain or backbone consisting of sugar-phosphate-sugar-phosphate-etc. repeating thousands or millions of times. Each sugar is attached to a nitrogenous base.
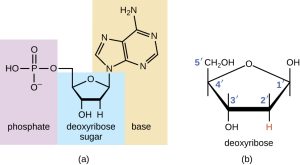
The numbering of carbons on the nucleotide molecule is a bit quirky. On the ring or rings of the nitrogenous bases, there are either 6 or 10 atoms in the ring; each is numbered. But then when we hook those up to a sugar, we would have to start the numbering at 7 or 10 depending on the base. That’s not workable. Rather, we restart the numbering by calling the carbon attached to the nitrogenous base carbon “1-prime” (written 1’). Going clockwise from there, the next carbon is 2’. This is the one that has either a –OH or –H attached to it in ribose or deoxyribose respectively. The next carbon is 3′, then 4’, then 5’.

Media Attributions
- U03-099 pyrimidines & purines © Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster is licensed under a CC BY (Attribution) license
- U03-100 text box © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-101a nucleosides cytidine © NEUROtiker is licensed under a Public Domain license
- U03-101b nucleosides guanosine © NEUROtiker is licensed under a Public Domain license
- U03-101c nucleosides uridine © NEUROtiker is licensed under a Public Domain license
- U03-101d nucleosides adenosine © NEUROtiker is licensed under a Public Domain license
- U03-101e nucleosides deoxythymidine © NEUROtiker is licensed under a Public Domain license
- U03-102 nucleotide parts © Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster is licensed under a CC BY (Attribution) license
- U03-098a text box © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license

