Noble Gases in the Periodic Table
Objective 2.6
2.6.1 Summarize the properties of noble gases and describe the orbital configurations that result in a noble gas.
 Noble gases, as described earlier, don’t participate in chemical reactions—not even with themselves. For example, argon is 1% of the atmosphere but we are unaware of it when we breathe because it doesn’t do anything except take up space.
Noble gases, as described earlier, don’t participate in chemical reactions—not even with themselves. For example, argon is 1% of the atmosphere but we are unaware of it when we breathe because it doesn’t do anything except take up space.
The chemical properties of an element depend on its electron orbital configuration. Filled orbitals represent a low-energy configuration, and to displace any electrons requires an input of energy. This is why the highly electronegative group 17 elements (F, Cl, Br, I) are so chemically reactive: they only lack one electron to achieve a filled-shell, low-energy state.
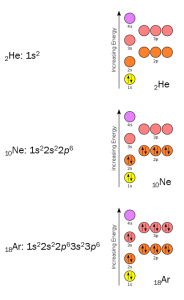
Noble gases are already in that low-energy state. For example, helium (2He) has only one orbital, the 1s. The 1s orbital can hold two electrons, and that’s how many a helium atom has: 2 protons, a variable number of neutrons (usually 2) and 2 electrons.
Neon (10Ne) has 2 electrons in the 1s orbital, 2 in the 2s orbital, and 6 in the 2p orbital. Thus, both level 1 (lowest energy) and level 2 (next-lowest) are filled. (Even though you don’t need to know this way of representing orbitals, chemists sometimes write this configuration as 1s22s22p6.)
Argon (18Ar) has 2 electrons in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in the 3s orbital, and 6 in the 3p orbital. Thus, its first-, second-, and third-level orbitals are all completely filled (1s22s22p63s23p6).
Krypton (36Kr), xenon (54Xe), and radon (86Rn) all have filled shells as well. All are in group 18 with He, Ne, and Ar, and all are noble gases. Radon is a radioactive gas which does not participate in chemical reactions. When uranium and radium decay, they emit alpha particles. Radon gas can accumulate in houses and expose the residents to radioactivity in the form of α radiation, which increases the incidence of cancer, especially lung cancer.
Media Attributions
- U02-028 periodic table © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. is licensed under a CC BY (Attribution) license
- U02-053 noble gases orbital diagrams © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license

