Lipids and the Cell Membrane
Objective 3.12
3.12.1 Define: phospholipid.
3.12.2 Describe how phospholipids are part of the mechanism for carrying lipids in the bloodstream.
3.12.3 Give examples of structures formed by phospholipid molecules.
3.12.4 Illustrate the properties of the phospholipid molecules in a lipid bilayer.
3.12.5 Give examples and recognize the cell surface markers with uniquely identify cells.
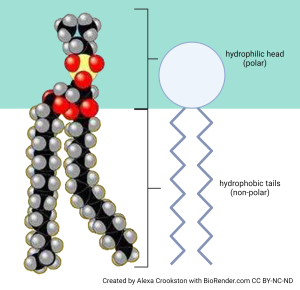 We’ll now consider a specialized lipid molecule called the phospholipid. A phospholipid consists of a polar, water-loving hydrophilic head group and two non-polar, water-hating hydrophobic tails. The heads are made of a variety of different chemical combinations (which are interesting, but not at this point in this course).
We’ll now consider a specialized lipid molecule called the phospholipid. A phospholipid consists of a polar, water-loving hydrophilic head group and two non-polar, water-hating hydrophobic tails. The heads are made of a variety of different chemical combinations (which are interesting, but not at this point in this course).
The tails are made of fatty acids whose properties can be changed. Again, the properties of these fatty acid tails are an interesting part of understanding how cells work but we’ll just nod to the concept now and perhaps study the details in other courses.
Phosphorus and oxygen are highly charged. In contrast, fatty acid tails do not have many free electrons, since most of the electrons from hydrogen atoms are in a stable relationship with carbon atoms.
Because they have both polar and non-polar parts, we call phospholipids amphipathic (literally, “both kinds of experience”). The amphipathic nature of phospholipids gives them some interesting properties we’ll study now.
Soaps (surfactants) are examples of amphipathic molecules. In both soaps and phospholipids, the polar head at one end of the molecule likes to be in a water environment (we say it is hydrophilic). The non-polar tails at the other end of the molecule “hate” water (we say they are hydrophobic).
In general, we use the terms hydrophilic and polar interchangeably. We use the terms hydrophobic and non-polar interchangeably. Notice the red oxygen atoms making up an important part of the polar heads, while teal hydrogen atoms and black carbon atoms are the major constituents of the non-polar tails.
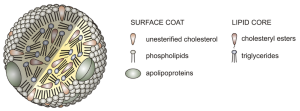
In a watery environment like the blood of humans, hydrophobic phospholipid tails hate water and point themselves away from it (as is their nature); hydrophilic phospholipid heads love water and point themselves towards it. Thus they come to surround a core of lipids that just basically hate water (the triglycerides and cholesteryl esters). On the outside, there is a sort of “candy shell” that has the head groups, cholesterol with its oxygen pointed out, and proteins called apolipoproteins. (“Apo–” means “lacking”, so when they join with lipids, they’re called lipoproteins.)
Lipoproteins are the lipid carriers in blood. They differ in density, and are believed to have different effects on health. Lipid is less dense than water; that is why oil floats on water. If the lipid content of the carrier is high, the resulting complex is called very-low density lipoprotein (VLDL). This is believed by medical scientists to be the most damaging form of lipoprotein. VLDL is deposited in adipose (fat) tissue.
Low-density lipoprotein (LDL) has an intermediate lipid content.
High-density lipoprotein (HDL) has the highest protein content, and the lowest lipid content. It is believed to exert a protective effect. HDL is increased by exercise.
Because phospholipids are amphipathic, they self-assemble into different structures when placed in water. They might form balls called micelles, with the non-polar tails pointing in and the polar heads on the outside. This is the form of lipid we see in salad dressing when we mix lipids (oil) and acetic acid (vinegar). Micelles are just big enough to scatter light and so make the suspension look cloudy.
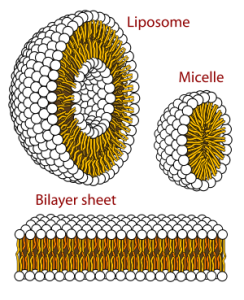
Liposomes are balls of phospholipid with a polar “shell” on the inside and the outside. These are used to deliver drugs to specific body tissues because they can be made to look like specific cells on the outside (more about this in Objective 15) and can hold water-soluble drugs on the inside without exposing all the cells of the body to the drug. The liposome attaches to specific cells and delivers the drug intracellularly only to its targets through a process called endocytosis, which we’ll discuss in Unit 4.
Unit 4 is all about phospholipids forming a bilayer, a sheet arrangement where the polar heads of phospholipids are pointing towards the watery environment inside and outside the cell, while the non-polar, hydrophobic tails self-associate with one another away from the water molecules they hate so much.
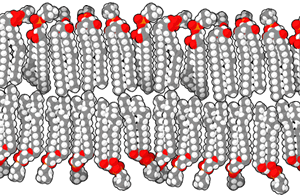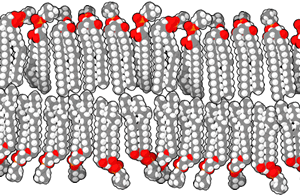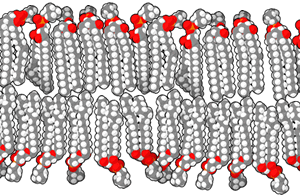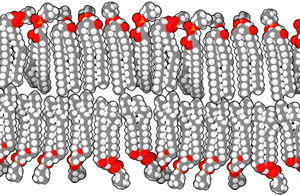
Phospholipids are formed when two fatty tails (carbon and hydrogen only) are joined by a glycerol molecule to a phosphate-containing “head group”.
Choline is a common part of head groups, but many other types of molecular groups can be found here. They are all rich in polar atoms, so they are hydrophilic.
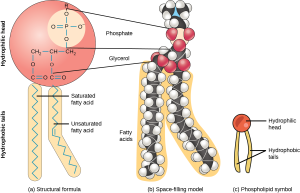
The tails, of course, being made up of only carbon and hydrogen, are hydrophobic.
The glycerol, phosphate and other parts of the head group associate with each other and prefer to be around water. The fatty tails will associate with other hydrophobic tails, as we will see later.
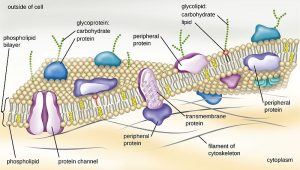
In Unit 4, we’ll see the specific properties of these cell membranes and study the details of a class of molecules called the glycolipids (branched sugar trees + two fatty acid tails) and glycoproteins (branched sugar trees + protein). These are “anchored” in the lipid bilayer by their hydrophobic regions (fatty acids or non-polar amino acid –R groups) and then protrude out into the watery extracellular environment to make the surface of each type of human cell unique.
For example, glycoproteins make up the cell surface markers called the human leukocyte (HLA) antigens. Nowadays, it’s more common to call these the major histocompatibility complex (MHC) glycoproteins even though technically MHC refers to the genes that code for these glycoproteins.
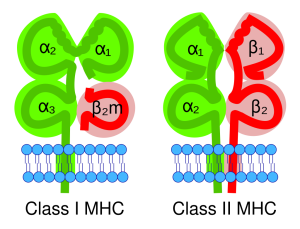
MHC glycoproteins come in two types, naturally enough called MHC-1 and MHC-2. Together, these uniquely identify each human being, so an MHC protein belonging to one person’s liver cells is different than an MHC protein belonging to another person’s. That’s where the name comes from: MHC proteins were discovered in the early days of human transplantation medicine when we found we couldn’t just slap Uncle Joe’s liver into your abdomen and expect you to live. This concept is called histocompatibility (histo–, “tissue” + compatibility). You have to share as many genes as possible with your potential donor in order to even have a chance of a transplant working. That’s why transplant teams study the MHC types of both the donor and recipient, trying to get the best “match” possible.
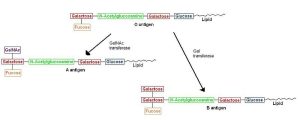
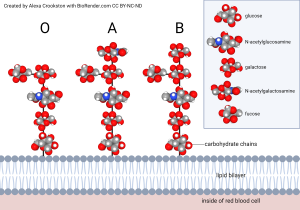
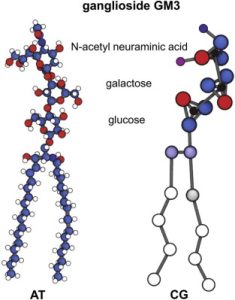 Polysaccharides attached to two fatty acids are the basis for the ABO blood grouping. Two of the monosaccharides involved are familiar to us from our previous discussions: glucose and galactose. The others are unfamiliar (n-acetylglucosamine; n-acetylgalactosamine; fucose) and we don’t need to learn them. This is the part of the molecule that is termed “glyco–”. The part called “–lipid” is comprised of two fatty acid tails, shown as blue carbons and white hydrogens in the top diagram and a squiggly yellow line in the bottom diagram. One of the monosaccharides involved is familiar to us from our previous discussion: galactose. The others are unfamiliar (N-acetylglucosamine; fucose), and we don’t need to learn them. The composition of polysaccharides and the branching pattern make each of these three molecules unique. Our immune system may recognize any foreign branching pattern (the A antigen and/or B antigen) as foreign. Every individual has red blood cells coated with the glycolipid called the O antigen so type O blood does not cause a major reaction in anyone.
Polysaccharides attached to two fatty acids are the basis for the ABO blood grouping. Two of the monosaccharides involved are familiar to us from our previous discussions: glucose and galactose. The others are unfamiliar (n-acetylglucosamine; n-acetylgalactosamine; fucose) and we don’t need to learn them. This is the part of the molecule that is termed “glyco–”. The part called “–lipid” is comprised of two fatty acid tails, shown as blue carbons and white hydrogens in the top diagram and a squiggly yellow line in the bottom diagram. One of the monosaccharides involved is familiar to us from our previous discussion: galactose. The others are unfamiliar (N-acetylglucosamine; fucose), and we don’t need to learn them. The composition of polysaccharides and the branching pattern make each of these three molecules unique. Our immune system may recognize any foreign branching pattern (the A antigen and/or B antigen) as foreign. Every individual has red blood cells coated with the glycolipid called the O antigen so type O blood does not cause a major reaction in anyone.
Media Attributions
- U03-120 Phospholipid head and tails © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U03-121 Structure_of_a_Lipoprotein © AntiSense is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-122 Phospholipids_aqueous_solution_structures.svg © LadyofHats, Villarreal, Mariana Ruiz is licensed under a Public Domain license
- U03-123 Lipid_bilayer_section © Bensaccount is licensed under a Public Domain license
- U03-124 phospholipid © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U03-125 PlasmaMem © Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster is licensed under a CC BY (Attribution) license
- U03-126 mhc molecules © Christensen, Zachary P. is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-127 glycoprotein © Shorthouse, David; Hedger, George; Koldsø, Heidi; Sansom, Mark S.P. is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U03-127b A and B antigens © Tmlew is licensed under a Public Domain license
- U03-128 Glycolipid coating red blood cell © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

