Lipids
Objective 3.5
3.5.1 State the features of lipids.
3.5.2 Name examples of biologically important lipids.
We’ll next turn to a second class of biological molecules, the fats or lipids.
Like carbohydrates, lipids generally contain carbon, hydrogen and oxygen.
Some lipids include phosphorus, nitrogen or sulfur atoms but these are not a major constituent of lipids.
Lipids are rich in carbon and hydrogen, and therefore have less oxygen. Remember from Unit 2 that the electronegativity of hydrogen and carbon are almost equal. Because they pull electrons about equally strongly, they tend to form covalent bonds where there is equal sharing of electrons.
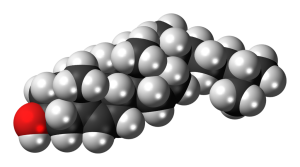
| cholesterol | 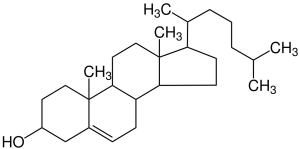 |
 |
| a free fatty acid | 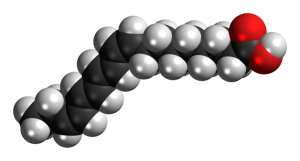 |
|
| a triglyceride | 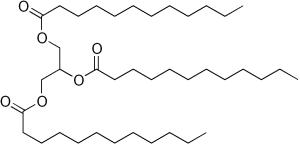 |
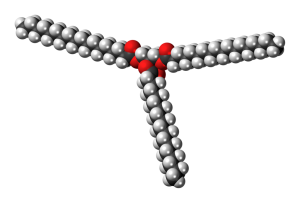 |
| a phospholipid | 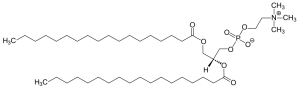 |
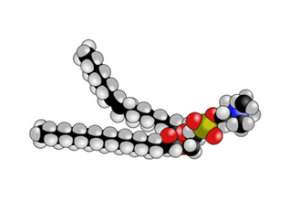 |
Equal sharing of electrons means fewer opportunities for hydrogen bonding. These molecules are termed non-polar because their bonds are covalent instead of polar covalent. Fewer opportunities for hydrogen bonding means fewer interactions with water. Lipids generally repel water (we say they are hydrophobic, which literally means “water-fearing”).
Lipids are:
- non-polar
- hydrophobic
- unable to form hydrogen bonds, therefore are insoluble in water (“oil and water don’t mix”)
These are three different ways of saying the same thing.
 We’ll next look at some subcategories of lipids. One major category are lipid molecules based on the structure known as cholesterol. These are collectively called steroids. Steroids in the human body include cholesterol itself; the sex steroids (estrogens and testosterone); the stress hormone cortisol; and vitamin D.
We’ll next look at some subcategories of lipids. One major category are lipid molecules based on the structure known as cholesterol. These are collectively called steroids. Steroids in the human body include cholesterol itself; the sex steroids (estrogens and testosterone); the stress hormone cortisol; and vitamin D.
Fatty acids are the basic building blocks of many lipids. They are made up of long chain molecules rich in carbon and hydrogen.
Fatty acids come in many different varieties. The carbon backbones vary from 4 to 20 carbons strung end-to-end, and with hydrogens filling the unused bonds on the carbon atoms. Each ends in a carboxyl (–COOH) group, which is where the “acid” part comes in.
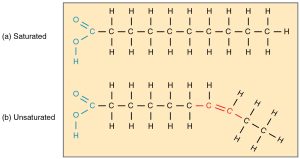
Saturated fatty acids are those where all the carbons are filled, or saturated, with hydrogen atoms.
When double bonds form between carbon atoms, fewer hydrogens can bond to carbons, and an unsaturated fatty acid results. The double-bonded carbon also puts a “kink” into the regular zigzag pattern of the carbon backbone. Because saturated fatty acids “pack” more closely (in the absence of kinks), they are solid at room temperature. Butter is an example of a saturated lipid. Polyunsaturated fatty acids have multiple kinks that keep the fatty acid molecules from closely packing, and so polyunsaturated lipids (like olive oil) are liquid at room temperature.
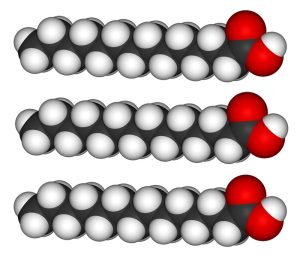
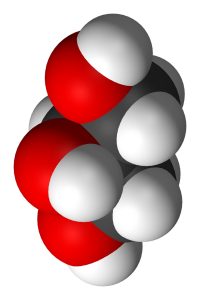
Three fatty acid chains can be added to a molecule of glycerol to form triglycerides. The fatty acid chains do not need to be of the same size or saturation; any combination is possible.
 |
+ |  |
= | 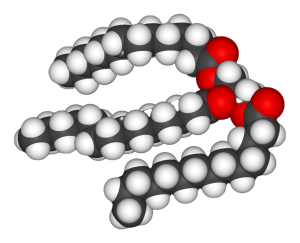 |
| three fatty acids | glycerol | triglyceride |
Recall that the formation of triglycerides from three fatty acid chains and a glycerol molecule is an example of a dehydration synthesis.
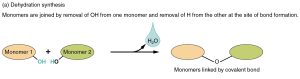
Each fatty acid chain ends in a –COOH. The fatty acid’s terminal –OH and a –H on glycerol combine to release water (HOH) and the oxygen bridges the glycerol backbone to the fatty acid “tails”.
Media Attributions
- U03-064a.1 Cholesterol © Wesalius is licensed under a Public Domain license
- U03-064a.2 Cholesterol © Jynto is licensed under a CC0 (Creative Commons Zero) license
- U03-064b.1 Fatty Acid © Hannahmjohnson13 is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-064b.2 Fatty Acid © Jynto is licensed under a CC0 (Creative Commons Zero) license
- U03-064c.1 Triglyceride © Edgar181 is licensed under a Public Domain license
- U03-064c.2 Triglyceride © Jynto is licensed under a CC0 (Creative Commons Zero) license
- U03-064d.1 Phospholipid © Benff is licensed under a Public Domain license
- U03-064d.2 Phospholipid © LetyClavr adapted by J.D. Speth is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-067 U03-068 U03-69 Slide © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U03-070a Triglyceride © Benjah-bmm27 adapted by J.D. Speth is licensed under a Public Domain license
- U03-070b Triglyceride © Benjah-bmm27 adapted by J.D. Speth is licensed under a Public Domain license
- U03-070c Triglyceride © Benjah-bmm27 adapted by J.D. Speth is licensed under a Public Domain license
- U03-011 same as U02-106 Dehydration_Synthesis_and_Hydrolysis-01 © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by J.D. Speth is licensed under a CC BY-SA (Attribution ShareAlike) license

