Isotopes
Objective 2.3
2.3.1 Define isotope.
2.3.2 Describe how different isotopes of the same element are formed.
2.3.3 Distinguish between stable isotopes and radioisotopes.
2.3.4 Summarize and discuss the forms of energy which are emitted by radioisotopes: positron emission; alpha (α), beta (β), and gamma (γ) radiation.
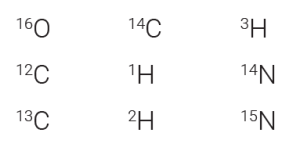
We indicate the mass of an isotope with a superscript number in front of the element name:
 Hydrogen-1 (1H) is the simplest atom. Hydrogen has an atomic number of 1, so we know that it always has one proton in its nucleus. For 1H, one is also the mass number, so there are zero neutrons in the nucleus. The number of protons and electrons is always equal for a non-charged atom, so there is also one electron. Because the number and arrangement of electrons determines the chemical properties of the atom, and because a neutral atom has the same number of electrons as it does protons, we can predict the chemical properties of an element by its atomic number (number of protons). We’ll see how this prediction works when we get to the periodic table in Objective 5.
Hydrogen-1 (1H) is the simplest atom. Hydrogen has an atomic number of 1, so we know that it always has one proton in its nucleus. For 1H, one is also the mass number, so there are zero neutrons in the nucleus. The number of protons and electrons is always equal for a non-charged atom, so there is also one electron. Because the number and arrangement of electrons determines the chemical properties of the atom, and because a neutral atom has the same number of electrons as it does protons, we can predict the chemical properties of an element by its atomic number (number of protons). We’ll see how this prediction works when we get to the periodic table in Objective 5.
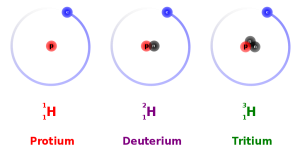
Remember, the number of neutrons found in the nucleus can vary without changing the chemical properties of the element. When the number of neutrons varies, we call these isotopes of the element. Because this changes the atomic mass (superscript before the symbol), the three isotopes of hydrogen are represented like this: 1H, 2H, 3H.
For example, hydrogen always consists of one proton and one electron. If we add a neutron, so that it consists of one neutron, one proton and one electron, the isotope is called hydrogen-2 (2H) or deuterium, which is sometimes represented as D. Deuterium plus oxygen makes up so-called heavy water (D2O), a compound used as a tracer in metabolic studies.
If we add one more neutron, the resulting hydrogen atom, with two neutrons, one proton and one electron, is called tritium (3H). Tritium is unstable and a tritium atom will fall apart, on average, after 12.3 years. We call this the half-life of tritium because over a 12.3 year period, there is a 50% probability a single tritium atom will fall apart. Another way of saying this is that half of the tritium atoms will fall apart in 12.3 years. When we create unstable isotopes of an atom, these are called radioisotopes.
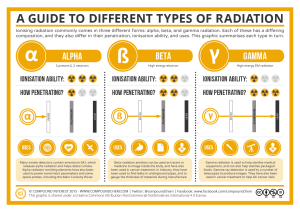
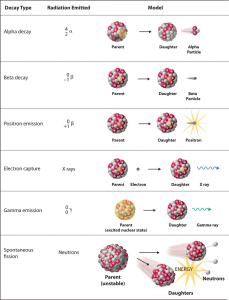
Unstable isotopes are called radioisotopes. Not all radioisotopes decay (fall apart) in the same way. Different forms of radioisotope decay are used in human medicine.
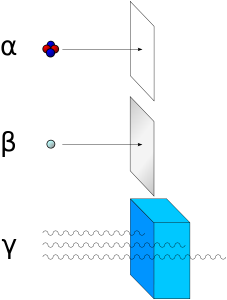
Alpha (α) particles are the same as helium nuclei. Helium is atomic number 2 and has an atomic mass of 4, but in an alpha particle the two electrons are missing.
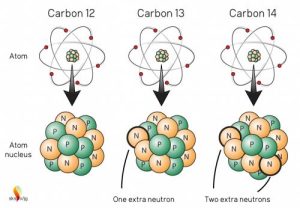
Beta (β) particles are electrons expelled at high energy from radioactive atoms. For example, 3H and 14C are beta emitters. Common isotopes of carbon are 12C, 13C, and 14C. 14C is unstable and falls apart, on average, every 5730 years. Like 3H, 14C emits an electron (beta particle or β) in the process of falling apart. The decay of 14C is the basis for radiocarbon dating in archeology.
A gamma (γ) particle (or gamma ray) is a high-energy packet of light energy (photon). Photons of lower energy (from highest to lowest) are x-rays, ultraviolet light, and visible light.
 One common mode of decay is by nuclear fission. In this case, more neutrons are found in the nucleus than can be supported, and the nucleus is unstable.
One common mode of decay is by nuclear fission. In this case, more neutrons are found in the nucleus than can be supported, and the nucleus is unstable.
One familiar kind of fission is the decay of 235U. This is the kind of uranium used in some nuclear weapons and reactors. When a neutron hits a 235U nucleus, the nucleus breaks apart, creating two new atomic nuclei and three neutrons. These three neutrons then collide with more 235U nuclei in a chain reaction, like the positive feedback loops we studied in Unit 1.2-16 2-17
Proton beams are used to treat prostate, brain and lung cancers. Neutron beams have also been used experimentally in cancer treatment. Particles called positrons, which are like electrons in all respects except they carry a positive charge, are used in positron-emission tomography (PET). (We call this antimatter: the positron is an antimatter electron. Antimatter is made up of antineutrons, antiprotons, and positrons.) The magnetic properties of hydrogen nuclei are used as the basis for nuclear magnetic resonance imaging, an old name for magnetic resonance imaging (MRI).
Technetium (Tc), atomic number 43, is the only element between atomic numbers 1 and 83 that does not occur naturally on Earth. Because it is not used anywhere in the body, it will not interact 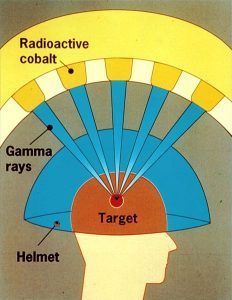 with the patient’s tissues. For this reason, it is used in medical diagnostic procedures. If we are referring to an atomic number, then that shows as a subscript: 43Tc. Remember the atomic number is equal to the number of protons (43 protons).
with the patient’s tissues. For this reason, it is used in medical diagnostic procedures. If we are referring to an atomic number, then that shows as a subscript: 43Tc. Remember the atomic number is equal to the number of protons (43 protons).
In nuclear medicine, we usually find atomic symbols with the mass number also indicated. For example, technetium-99m (the m stands for “metastable”, a concept which will not be explained here), or 99MTc, is the form of Tc used in medicine.
The superscript “99” before the element name tells us that 99 is the mass number (43 protons + 56 neutrons = 99) of the artificially created atom. In the periodic table we’ll see in Objective 5, the atomic mass is in parentheses, indicating there are no technetium atoms in nature.
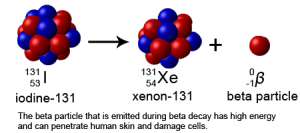
Other atoms commonly used in nuclear medicine include 47Ca (20 protons + 27 neutrons) to study bone loss and the location of tumor cells, 131I (53 protons + 78 neutrons) to destroy thyroid tissue, and 133Xe (54 protons + 79 neutrons) for respiratory and cerebral blood flow studies.

Media Attributions
- Superscripts © West, Jordan is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-014 Hydrogen_Deuterium_Tritium_Nuclei_Schmatic-en.svg © Hünniger, Dirk is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-016 CarbonIsotopes_med © Skeptical Science is licensed under a CC BY (Attribution) license
- U02-015 A-Guide-to-Different-Common-Types-of-Radiation © Compound Interest is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-017 Alfa_beta_gamma_radiation.svg © Stannered is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-018 notation_nuclear_reactions hutchins mod © UC Davis Chemwiki is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-019 Gamma_Knife_Graphic © Nuclear Regulatory Commission is licensed under a Public Domain license
- U02-020 Beta-decay © PerOX is licensed under a CC0 (Creative Commons Zero) license
- U02-021 isotopes in human medicine © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license

