Glycogen, Lipids, and Proteins as Energy
Objective 5.9
5.9.1 Explain how glycogen, lipids, and proteins are used as energy sources.
5.9.2 Name any metabolic by-products of their use.
Glycogen as Energy Storage
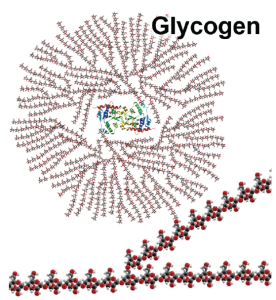 Excess glucose, not needed for cellular metabolism, is converted to glycogen (long, branching chains of connected glucose molecules). Glycogen is easily produced, easily broken down, and is stored primarily in the liver (up to 100 g, 400 Cal) and in skeletal muscle (up to 400 g, 1600 Cal). Cardiac muscle also stores glucose as glycogen, but only about 2 g. We can store a maximum of about 500 g of glycogen, which would produce about 2000 Calories (about 1 day’s worth of energy). When blood glucose gets low and more ATP is needed, glycogen is quickly broken down into individual glucose molecules, which are then available for cellular respiration.
Excess glucose, not needed for cellular metabolism, is converted to glycogen (long, branching chains of connected glucose molecules). Glycogen is easily produced, easily broken down, and is stored primarily in the liver (up to 100 g, 400 Cal) and in skeletal muscle (up to 400 g, 1600 Cal). Cardiac muscle also stores glucose as glycogen, but only about 2 g. We can store a maximum of about 500 g of glycogen, which would produce about 2000 Calories (about 1 day’s worth of energy). When blood glucose gets low and more ATP is needed, glycogen is quickly broken down into individual glucose molecules, which are then available for cellular respiration.
Lipids as Energy Storage
Excess glucose and fats, not needed for cellular metabolism or glycogen production (the glycogen tank is full), are converted and stored as lipids (fat, adipose tissue). The process isn’t as quick or easy as glycogen production and is a bit of a longer-term storage solution. Glucose is converted to glycerol, which is combined with three fatty acids to form a triglyceride molecule. Triglycerides are how our bodies store fat. One pound of triglycerides contains about 3500 Calories.
When cellular metabolism has exhausted available glucose and glycogen, triglycerides are mobilized from adipose tissue and broken into their original glycerol and three fatty acids. Since glycerol is a 3-carbon molecule, similar to pyruvic acid, we can stick it into the cellular respiration process in place of pyruvic acid, where it will lose a carbon and then get shoveled into the citric acid cycle by coenzyme A (CoA). The three fatty acids (long chains of carbon atoms) require a bit more “processing.” A reaction called beta-oxidation (β-oxidation) cleaves two carbons at a time from the fatty acid chains.
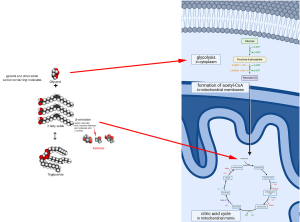
These 2-carbon molecules fit into the coenzyme A (CoA) shovel, just like acetyl groups, and are tossed into the citric acid cycle.
The process of generating glucose from a non-carbohydrate molecule (like lipids or proteins) is called gluconeogenesis (Greek gluco- “sweet,” Greek neo- “new,” and Greek genesis “create”) or “the creation of new glucose.” Sometimes the process proceeds all the way to the creation of a new glucose molecule (this usually occurs in the liver). Sometimes we stop short of that and feed the glucose precursors directly into the citric acid cycle (no reason to make them into glucose, only to break them down again to make ATP).
The complicated diagram is shown in a blue box because you don’t need to know it. Again, the essential information is that there is a process called gluconeogenesis which is essentially the reverse of glycolysis. Whenever we have extra 2- or 3-carbon molecules around (for example, amino acids; pieces of fatty acids and glycerol from lipids), we can assemble them back together into glucose for storage in the liver. Or we can feed them directly into the citric acid cycle as 2- or 3-carbon molecules.

Unfortunately, gluconeogenesis to power ATP production is not as efficient as using dietary glucose. A by-product of β-oxidation is ketones. There are three different ketone molecules, two of which are organic acids. We make a few ketones all the time, as we break down triglycerides, and they don’t pose a problem. However, in individuals with poorly managed diabetes mellitus, when they can’t get any glucose from blood into cells and so the cells rely entirely on triglycerides for energy production, ketone levels can get quite high. This results in ketosis (fatigue, performance decrease, weight loss) and can progress to ketoacidosis (excessive thirst, frequent urination, nausea/vomiting, abdominal pain, weakness/fatigue, shortness of breath, fruity breath smell, confusion).
The third ketone molecule is acetone, the solvent used in fingernail polish remover. As blood ketones increase, ketotic or ketoacidotic patients often smell like fingernail polish remover (because of ketone secretion into the breath and sweat). There are ketones in the urine and blood that can be detected with laboratory tests. These signs can prompt alert medical personnel to direct their clinical suspicion toward the correct diagnosis.
Proteins as an Energy Source
By now you may have realized that glycogen serves as our quick, easy to retrieve, and readily available source of glucose, while lipids fill the role of a more long-term glucose storage system (and also serve as padding and/or insulation until needed for energy). The remaining source of “stored” energy in the body is protein, which serves as our emergency back-up source, to be used only as a last resort.
The human body does not store significant amounts of energy as protein. Rather, our protein “storage” is located throughout the body as an integral component of cell structure, contractile filaments, channels, and carrier molecules. Using these proteins as an energy source damages cells and negatively affects numerous body processes. Thus, protein really is our energy source of last resort.
The breakdown of proteins from skeletal muscle and other tissues releases large amounts of amino acids. The 2-carbon backbone of amino acids can be fed directly into the citric acid cycle by coenzyme A. Unfortunately, gluconeogenesis using proteins is even less efficient than using triglycerides. Cleaving the 2-carbon backbone from an amino acid leaves the amino group (–NH2), which is converted to urea and excreted in urine (with other nitrogenous wastes). Some amino acid side chains are usable for energy metabolism and some are not, depending on their structure.
While it is true that proteins in foods have caloric value, in most cases dietary protein is broken down to amino acids which are then used to make new proteins that the body needs. This is much more efficient than making amino acids from scratch. Some amino acids (called essential amino acids) cannot be made in the body and must be part of the diet. Which of the 20 amino acids are essential varies depending on age and health status, but eight amino acids (isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine) must be part of the diet for all of us to maintain health.
The body is quite good at maintaining blood glucose level. The average adult needs about 2000 Calories per day to function and stay healthy. At any given moment, depending on how long it has been since our last meal, we could be burning glucose and storing glycogen and lipids, burning glucose released from glycogen, making glucose from lipids, or making glucose from protein. Blood glucose and glycogen are depleted about 12 hours after our last meal; that’s when we begin gluconeogenesis, first from lipids and, if we deplete our lipids, from protein. Regardless of which source of energy we are burning to generate ATP, blood glucose stays amazingly constant. Even after 40 days of fasting (starvation), blood glucose remains quite stable. However, free plasma fatty acids increase as fats are mobilized for gluconeogenesis and then decrease as they are depleted, and blood ketones go up as soon as we start gluconeogenesis (and keep going up…).
Organelles
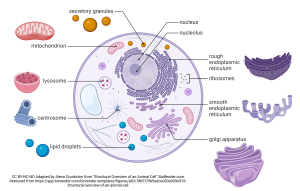
This drawing gives you an overview of the organelles in a mammalian cell.
In Objectives 2-8, we focused on cellular metabolism and the interplay between metabolic reactions in the cytoplasm (glycolysis) and mitochondrion (formation of acetyl CoA, the citric acid cycle, and the electron transport chain).
Now we’ll turn our attention to all the other organelles found within a human cell. We’ll start with the cytoskeleton (Objective 10), then consider the movement organelles (11), synthesis organelles (12), and finally discuss the storage and digestion organelles (13).
Media Attributions
- U05-023 glycogen composite © Häggström, Mikael adapted by Jordan West is licensed under a CC0 (Creative Commons Zero) license
- U05-024 metabolism of fats © Hutchins, Jim is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U05-025 gluconeogenesis text box © Hutchins, Jim is licensed under a Public Domain license
- U05-027 Structural Overview of an Animal Cell © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

