Functional Groups and Polyatomic Ions
Objective 2.14
2.14.1 Identify, on a formula, line diagram or space-filling model, each of the functional groups and polyatomic ions listed here.
Especially in biochemistry, we save time by naming combinations of atoms that occur quite frequently. The formulas for these are listed on the following table along with their names.
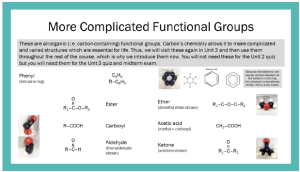
We are introducing a new symbol here. Chemists use the letter R to indicate any single atom or grouping of atoms, if its composition doesn’t really matter. These are also called “R groups”. Notice that R is not found in the periodic table, so it can’t be confused with anything else. I like to think that chemists talk like pirates: whenever they don’t know what to say, they say, “arrrrrrrrr”.

These may appear on the exam in any of the forms seen on these pages: formulas, line diagrams, or photographs of space-filling models. This is a good time to make flashcards, because it’s a straight-up memorization task to learn all 10 of them.
In organic chemistry (carbon chemistry) and biochemistry (life chemistry), we commonly refer to functional groups when describing complex molecules.
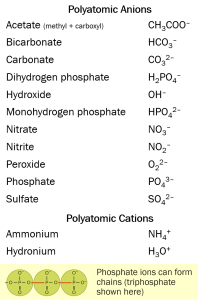
Inorganic chemistry also has a similar concept called polyatomic ions. Combinations of certain ions “travel together” to form what are termed ionic compounds or polyatomic ions. We have already seen one example of this: bicarbonate (HCO3–), a common polyatomic ion and electrolyte in blood. When it combines with Na+, it forms sodium bicarbonate (NaHCO3). Common ionic compounds are listed below.
Hydroxyl (–OH) or its ionic form hydroxide (OH–) appears in both tables. We’ve used many of these in the discussion above (e.g. bicarbonate) and so you might know many of these already.
There is no reason why two polyatomic ions can’t combine, and they do. For example, household ammonia (ammonium hydroxide) is NH4OH. Scientists and bakers use ammonium bicarbonate (NH4HCO3 or NH5CO3), called hartshorn in baking.
Media Attributions
- U02-099 functional groups © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-100 functional groups © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-101 complicated functional groups blue box © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-102 replacement polyatomic ions © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license

