Enzymes
Objective 3.4
3.4.1 Discuss the mechanism of action of enzymes.
 Enzyme pockets are the next category of pockets we’ll examine in detail. Remember that in Unit 2 we quoted Tom Cech as saying, “chemistry occurs when two molecules collide with the right orientation and velocity.” We can get tired of waiting around for the molecules to collide randomly to do this, especially when we’re trying to manage a cell with tens of thousands of chemical reactions taking place.
Enzyme pockets are the next category of pockets we’ll examine in detail. Remember that in Unit 2 we quoted Tom Cech as saying, “chemistry occurs when two molecules collide with the right orientation and velocity.” We can get tired of waiting around for the molecules to collide randomly to do this, especially when we’re trying to manage a cell with tens of thousands of chemical reactions taking place.
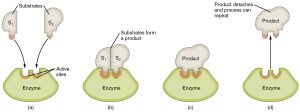
One way of speeding up this process is to use a biological catalyst called an enzyme. These are proteins that speed up (catalyze) chemical reactions. The molecule(s) being acted upon by an enzyme are called the substrate(s) and the molecules being created are called the product(s). The enzyme is usually named after the substrate followed by the ending –ase. For example, in the following pages, we’ll look at carbonic anhydrase and sucrase which are enzymes that act on carbonic acid and sucrose, respectively. The enzyme acetylcholinesterase breaks apart the nerve signaling molecule acetylcholine (Units 10 and 13) at an ester linkage.
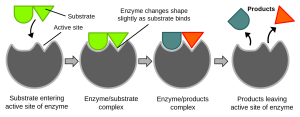
A typical human cell carries out tens of thousands of metabolic processes. Each of these reactions is speeded along by an enzyme of one sort or another. It’s easier to get those molecules to collide if we hold them in place using a pocket lined with the right amino acid –R groups to hold that molecule, or those molecules, in place.
We could wait a long, long time for a water molecule and a carbon dioxide molecule to bang into each other with the correct orientation and velocity. It would be better if we could align their collisions. The energy needed to make this alignment happen is called the activation energy. If we have to wait for a collision to occur randomly, it takes more energy for that chemical reaction to take place (a higher “hill” in the middle of the diagram). If we can hold the reactants in such a way that they collide more efficiently, less energy is required (a lower “hill” in the middle of the diagram).
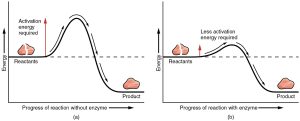
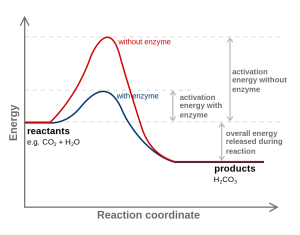
The other side of the energy “hill” — the downhill side — is the energy that’s released when the new bonds form.
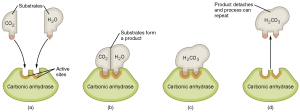
We will examine this process in detail with the enzyme carbonic anhydrase, which catalyzes the reaction between carbon dioxide and water that we first studied in Unit 2.
If we can get CO2 and H2O to sit next to each other at the dinner table, we’re pretty sure we can get them talking to each other and they might even form a chemical bond. (Two single people sometimes have chemistry—see what I did there?) We are simply managing the process of the molecules colliding with the right orientation and velocity. This management requires some energy (the activation energy) but the activation energy is less with the enzyme than without it.
The energy tied up within the chemical bonds of the CO2 and H2O molecules (the reactants), and the energy tied up within the chemical bonds of the H2CO3 molecule (the product) are physical constants that have to do with the nature of the chemical bond. We can’t change those. All we can change is their orientation to help a chemical reaction to take place faster by lowering the activation energy of the reaction.

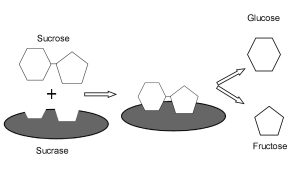
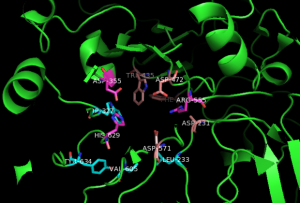
Another well-studied enzyme is sucrase, the enzyme which breaks down table sugar (sucrose) into its parts, which we’ll see later are called glucose and fructose. To do this, sucrase makes a pocket out of several of its amino acids (Asp355, Trp435, Asp472, Arg555, Asp231, Leu233, Asp571, Val605, Tyr634, His629, and Trp327). Notice these aren’t adjacent in the primary sequence but the secondary and tertiary structure of the sucrase protein brings these amino acids together into a pocket which is specially designed to hold onto the sucrose in my Cap’n Crunch cereal. Then, the pocket changes shape slightly which breaks a chemical bond, and then changes shape again to release the products (in this case, glucose and fructose).
Enzymes generally work pretty well on their own, but like most things, there’s also room for improvement. Because the shape and chemical nature of the pocket is so critical, anything that helps form a better pocket helps increase the efficiency of the enzyme by further reducing the activation energy. The chemicals which make enzymes work better are called cofactors or coenzymes.
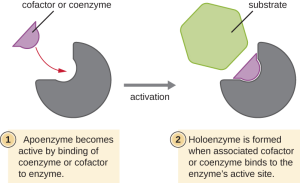 |
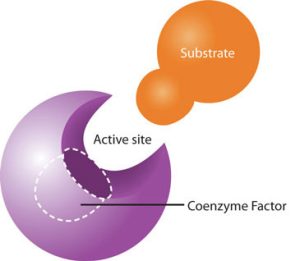 |
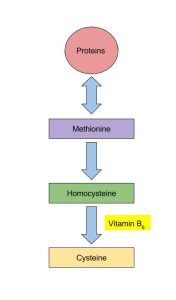 You’re probably familiar with many of these under other names. For example, vitamins are mostly cofactors which help enzymes work better. Vitamin B-6 is a coenzyme for over 100 different enzymes.
You’re probably familiar with many of these under other names. For example, vitamins are mostly cofactors which help enzymes work better. Vitamin B-6 is a coenzyme for over 100 different enzymes.
You might remember a story from grade school of sailors’ teeth falling out because they didn’t have access to fresh fruit. (The disease is called scurvy.) You may have wondered, as I did, what the heck fruit and teeth had to do with each other. The connection is through a cofactor called vitamin C and the synthesis of the major protein of connective tissue (including the connective tissue holding teeth and gums together): a protein called collagen. We’ll see a lot of different kinds of collagen in Unit 7 when we zoom in on connective tissue. The enzymes which carry out collagen synthesis are more efficient when vitamin C is present.
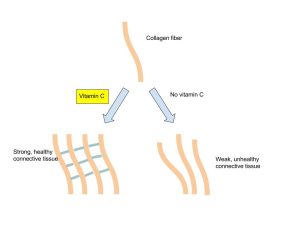 Vitamin K (German: “koagulation”) is needed for making blood clotting factors. Without vitamin K, these clotting factors are harder to make, and blood doesn’t clot as fast.
Vitamin K (German: “koagulation”) is needed for making blood clotting factors. Without vitamin K, these clotting factors are harder to make, and blood doesn’t clot as fast.
We’ll also see a number of coenzymes which speed up and smooth the process of metabolism when we study those processes in Unit 5.
Media Attributions
- U03-051 Carboxypeptidase_A © Lijealso is licensed under a Public Domain license
- U03-052 enzymatic reaction steps © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U03-053 Enzymes-01 © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U03-054 Carbonic_anhydrase_reaction_in_tissue.svg © Fvasconcellos is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-055 enzymatic reaction steps carbonic anhydrase © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U03-056 carbonic acid bicarb © Hutchins, Jim (Own Work) is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-057 sucrase © NuFS, San Jose State University is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-059 Induced_fit_diagram enzyme.svg © ImranKhan1992 is licensed under a CC0 (Creative Commons Zero) license
- U03-058 sucrase isomaltase_active_site © Areid3 is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-060 apoenzyme holoenzyme © Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster is licensed under a CC BY (Attribution) license
- U03-061 cofactor enzyme © University of Hawai‘i at Mānoa Food Science and Human Nutrition Program is licensed under a CC BY (Attribution) license
- U03-062 vitamin b6 © Calabrese, Allison is licensed under a CC BY (Attribution) license
- U03-063 vitamin c collagen © Calabrese, Allison is licensed under a CC BY (Attribution) license

