Energy and the Chemical Bond
Objective 2.10
2.10.1 Illustrate how energy is stored in, and released from, chemical bonds.
2.10.2 Explain the relationship of electrons in covalent bonds, polar covalent bonds, ionic bonds, and hydrogen bonds.
2.10.3 Classify the relationship between the energy in these bonds and the strength of the bond.
Energy comes in two forms: potential and kinetic.
Potential energy, like a coiled spring, is stored energy that is not (yet) able to do work.
Kinetic energy is the energy of movement. When you are riding in a ski lift to the top of a mountain, you are moving: this is kinetic energy. When you pause at the top and look down, you have turned the kinetic energy of the ski lift into potential energy. When you begin to slide down the hill, that potential energy is turned back into kinetic energy.
Energy is conserved. It probably won’t surprise you to know that this concept is called The Law of Conservation of Energy. This Law says chemical energy can change forms (from kinetic to potential, and back again) but it cannot be gained or lost.
(You may have heard of Einstein’s famous equation: E = mc2. The reason this was revolutionary is that it violates the Law of Conservation of Energy. Nuclear energy, as contrasted with chemical energy, can be converted to matter and back into energy again. Hence the nuclear bomb.)

When it is released to do work (like lifting a dumbbell) it is converted to kinetic energy. Since this is what cells of your body spend most of their time doing, we will study the general rules for converting food to cellular chemical bonds and converting cellular chemical bonds to work in Units 3 and 5, and then amplify on those concepts throughout the course.
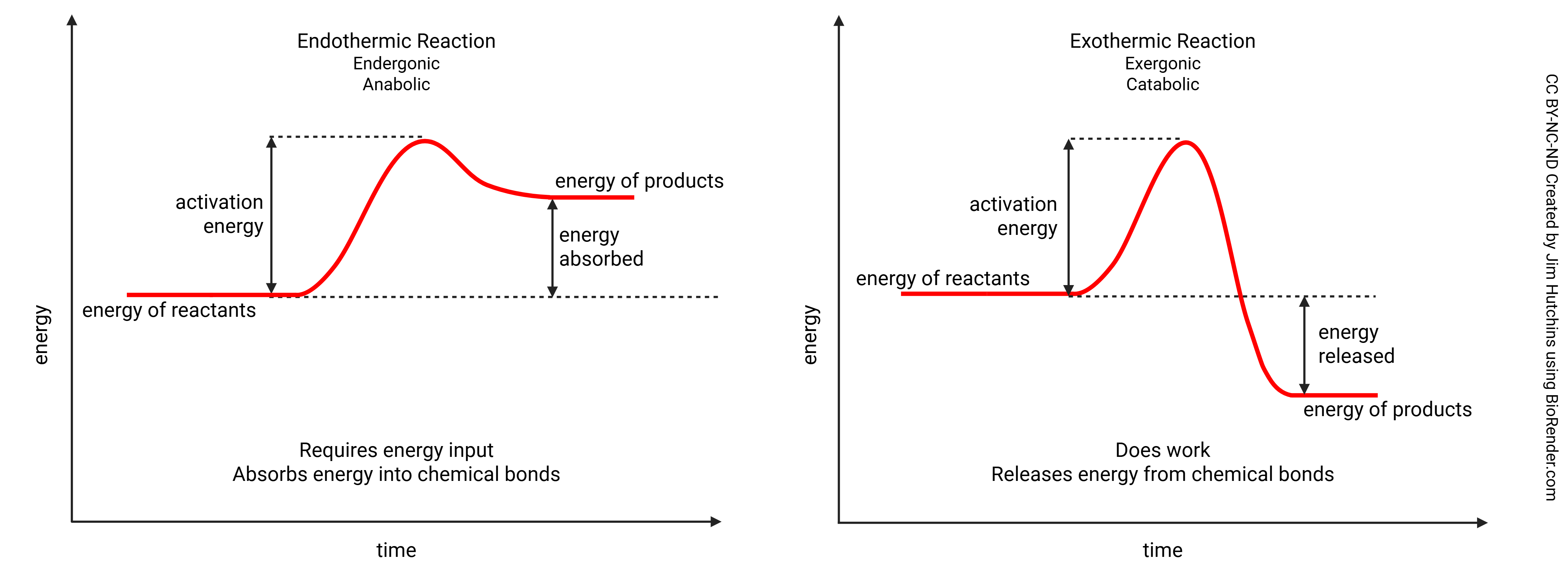
Chemical reactions that convert kinetic energy to potential energy of chemical bonds are called endergonic which is roughly equal to endothermic (endo–, inner; –therm, temperature). The potential energy is hidden within these chemical bonds (i.e. we say the reaction absorbs energy).
In biology, endergonic or endothermic reactions are roughly equal to anabolic reactions, our name for chemical reactions which form chemical bonds.
Chemical reactions which release the potential energy of molecules from their bondage are called exergonic or exothermic. Remember that the kinetic molecular theory says increasing temperature increases the kinetic energy of molecules, so this type of chemical reaction converts potential energy to kinetic energy.
 Our lab director, Pamela, and the male firefly would like me to remind you here that in rare circumstances, the excess energy from a chemical reaction is given off as visible photons rather than as heat. This happens in Pamela’s glowstick demo and in bioluminescent (“living light-emitting”) animals such as the firefly pictured here.
Our lab director, Pamela, and the male firefly would like me to remind you here that in rare circumstances, the excess energy from a chemical reaction is given off as visible photons rather than as heat. This happens in Pamela’s glowstick demo and in bioluminescent (“living light-emitting”) animals such as the firefly pictured here.
In biology, exergonic or exothermic reactions are roughly equal to catabolic reactions, our name for chemical reactions which break chemical bonds.
In most of the chemical reactions we’ll study, the energy is turned into work. For example, the breaking of chemical bonds allows muscles to contract, and a weightlifter can raise a barbell over his head in a “clean and jerk” maneuver.
Notice that the Greek word εργου (pronounced “ergon”) is work, which is where exergonic comes from. When iron particles rust, they give off heat in an exothermic reaction, which is why powdered iron is used in handwarmers, and why the handwarmers last longer if they are inside a glove (where there is less oxygen) than out on a table.
The three Laws of Thermodynamics tell us that no reaction is 100% efficient. In a biological system, the chemical bond energy that isn’t turned into work is turned into heat.
Heat production is a side-effect which has been harnessed by our body. For example, if we are cold, we contract our muscles without doing work; this produces waste heat, which helps us to stay warm. That’s why we shiver when we’re cold.
It’s also why heat management becomes a problem if we do work in hot weather. In that case, we may have a harder time shedding the waste heat—we might overheat. You’re probably sick of me reminding you of the Tom Cech quote: chemistry happens because molecules and atoms collide together with the right orientation and velocity. But we need to use that idea again. Remember that in the kinetic molecular theory, molecules and atoms are colliding. Sometimes, their collisions have the right orientation and have just enough energy (velocity) that their electrons have no choice to interact, and sometimes, rearrange themselves. These electron rearrangements are where chemical bonds are formed: where we convert kinetic energy (velocity) to potential energy (a chemical bond).
In an endothermic reaction, energy must be used to build a chemical bond. Notice the energy of the products is higher than the energy of the reactants. Endothermic reactions are storing energy in the chemical bonds that are built.
In an exothermic reaction energy is released as a bond is broken. Notice that activation energy is still needed to break the bond. As energy is released, the energy of the products is lower than the energy of the reactants.
Either the orientation of molecules or their velocity requires more energy than will eventually be represented in the resulting chemical bond. This energy, which you can think of as a kind of “hill” the electrons in the molecules need to “climb”, is called the activation energy. We will see ways of lowering the activation energy in Unit 3. We can lower the activation energy (i.e. by making sure the molecules are correctly oriented) but we can’t change the potential energy in the chemical bonds of the reactants, nor can we change the potential energy in the chemical bonds of the products.
There are different kinds of covalent bonds, with different strengths.
Covalent bonds result when atoms share electrons. For example, when two hydrogen atoms share both of the electrons they hold in common, it’s called a covalent single bond. Carbon (6C) always makes four bonds. It can complete its shell by releasing four electrons (to be like He), or by capturing four electrons (to be like Ne): 2He (6 – 4 = 2) or 10Ne (6 + 4 = 10) are the nearest noble gases with completed shells. For example, if C shares four electrons with four H atoms, four covalent single bonds are formed, as in methane (CH4). Equal sharing of electrons, in quantum terms, means there’s an equal probability of finding the electron near the carbon nucleus or near the hydrogen nucleus.
If H shares its single electron with another H, a covalent single bond is formed as we saw previously. A single bond is usually indicated by a single line:
H — H
A covalent double bond is when four electrons are shared equally. For example, each O atom wants to gain two electrons to complete its shell; when each oxygen atom shares two electrons, for a total of four electrons shared between two atoms, it can achieve the same goal. A covalent double bond is symbolized by a double dash:
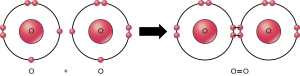 O = O
O = O
O = C = O
A covalent triple bond is when six electrons are shared equally. For example, each nitrogen atom wants to gain three electrons to complete its shell; when each nitrogen atom shares three electrons, for a total of six electrons shared between two atoms, they can achieve the same goal. A triple bond is symbolized by three lines:
N ≡ N
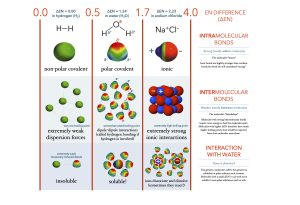
In polar covalent bonds like those in H2O, the oxygen nucleus is more highly electronegative so it has greater electron pulling ability. In quantum terms, there is a higher probability of finding the electron near the oxygen nucleus than near the hydrogen nucleus. The difference in probability is observed as a partial negative (higher probability of finding a negatively-charged electron) or partial positive charge (lower probability of finding a negatively-charged electron) in different parts of the molecule. In science, the lower-case Greek letter delta (δ) is used to mean “partial” so a partial positive charge is symbolized δ+ and a partial negative charge is symbolized δ–.
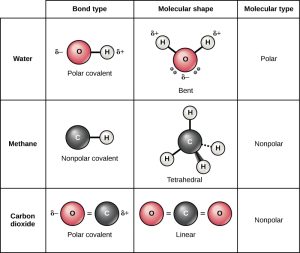
In HCl, there is a greater chance of finding the electron near the highly electronegative Cl nucleus so the Cl end of the molecule is labeled δ– while there is a smaller chance of finding the electron near the H end of the molecule so that end is labeled δ+.
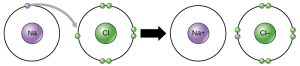
Ionic bonds result from the complete removal or addition of electrons to an atom. For example, there is a difference of 2.2 units between Na and Cl. The highly electronegative Cl atom just straight-up takes the electron; now 17Cl has 17 protons and 18 electrons for a net charge of –1 (symbolized Cl–). The weakly electronegative Na atom cannot hold onto its valence electron (nor does it want to, since it can complete an energy level by giving it away) so 11Na now has 11 protons and 10 electrons for a net charge of +1 (symbolized Na+).
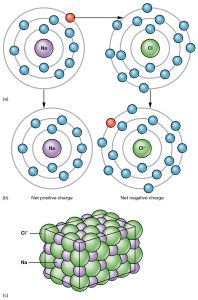
In a crystal of Na+Cl–, there is an opposite-charge attraction between the Na+ and Cl– ions, and it’s this charge attraction that holds the ions together. In other words, they are joined by an ionic bond.
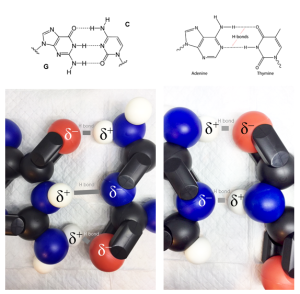
Hydrogen bonds result when a hydrogen atom gets into a relationship with oxygen, nitrogen, or sulfur, each of which results in an electronegativity difference of 0.5 to 1.7, the range for formation of polar covalent bonds within the molecule. Remember that polar covalent bonds result in a δ– near the more electronegative molecule (O, N, or S) and a δ+ near the H. These partial positive and negative charges attract each other like ionic bonds, but in a weaker sort of way.
 Of the bonds we’ve discussed, covalent bonds are the strongest. Of covalent bonds, triple are stronger than double, and double covalent bonds are stronger than single covalent bonds. Ionic bonds are the next strongest type. Hydrogen bonds are the weakest type of bonds that we’ve discussed.
Of the bonds we’ve discussed, covalent bonds are the strongest. Of covalent bonds, triple are stronger than double, and double covalent bonds are stronger than single covalent bonds. Ionic bonds are the next strongest type. Hydrogen bonds are the weakest type of bonds that we’ve discussed.
Summarizing bond strength:

Media Attributions
- U02-063a bond energy graphic © Hutchins, Jim
- U02-065 and U2-066 and U05-005 Energy Profile of a Chemical Reaction © Hutchins, Jim is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-064 Photinus_pyralis_Firefly_glowing © Art Farmer is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-067 double bond © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U02-068 types of bonds © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U02-069 ionic bonding © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U02-070 ionic bond © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-071 hydrogen bonds snatoms © Hutchins, Jim is licensed under a CC BY-ND (Attribution NoDerivatives) license
- U02-071a bond strength graphic © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-072 electronegativity infographic © Kennedy, James is licensed under a CC BY-SA (Attribution ShareAlike) license

