Endergonic and Exergonic Reactions
Objective 5.3
5.3.1 Compare and contrast endothermic/endergonic/anabolic reactions vs exothermic/exergonic/catabolic reactions.
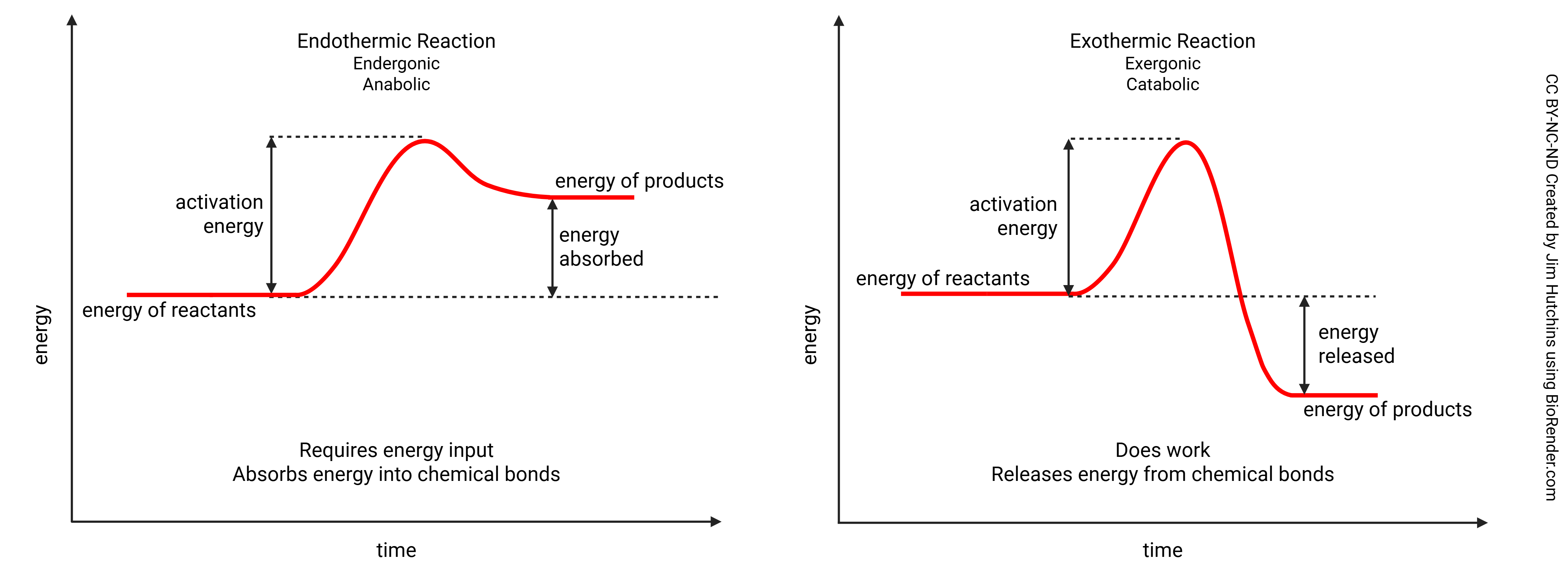
In chemistry, there are two basic types of chemical reactions: 1) exothermic or exergonic reactions, which release energy from chemical bonds, and 2) endothermic or endergonic reactions, which require an input of energy which is then stored in chemical bonds. (Both types require activation energy to get started.)
In biochemistry and physiology, we use the terms anabolic and catabolic in place of endergonic and exergonic, respectively. Anabolic reactions form bonds and generally absorb energy into those bonds, while catabolic reactions break bonds and generally release energy from those bonds.
The process of making and breaking bonds to release, capture, store, and then use energy inside our body cells is called metabolism. The basic metabolic process has two parts that occur in an ever-recurring cycle. One part, anabolism (an endergonic reaction), involves putting smaller molecules together to make larger molecules; it requires energy that is then stored in the newly created chemical bonds. (Some athletes have been known to take anabolic steroids, which promote the formation of complex proteins in muscle tissue, making muscle cells—and, therefore, muscles—larger.) The other part, catabolism (an exergonic reaction), occurs when we break down large molecules into smaller molecules; it releases the energy as the bonds are broken.
![]()

ATP is the “connection” between the anabolic and catabolic reactions in body cells. As catabolic reactions break complex molecules (carbohydrates, proteins, lipids) into smaller molecules (sugars, amino acids, fatty acids), the energy released is captured and stored in ATP molecules. As anabolic reactions need energy to put smaller molecules together into complex molecules, the required energy comes from ATP.
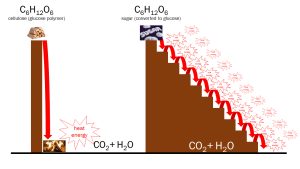
Metabolism is a carefully controlled burning process (oxidation in chemical terms). If you burn sugar in a flame, it is converted to CO2 and H2O and releases energy as heat (a catabolic reaction). The same thing happens when sugar is “burned” during cellular metabolism to make ATP. The difference is that in metabolism, each step is carefully controlled so that as much energy as possible is captured and stored in the high-energy bonds of ATP. Any energy that isn’t transferred to ATP is released as heat. The end-products are still CO2 and H2O, whether the sugar is burned in a fire or metabolized in a cell.
When a cell needs energy to power the work of the cell, it “reaches for its wallet” and pulls out some ATP energy currency. It takes one ATP and breaks the terminal phosphate group (-PO4) off the end, releasing the previously-stored energy from the now-broken bond. Because ATP has three phosphate groups and one has been removed, the result is an adenosine diphosphate (ADP) molecule.
When we consume food and “burn” the carbohydrates (catabolism), we turn ADP back into ATP by putting that phosphate group back on and storing the released energy in the newly-reformed bond between those last two phosphates. Recall from the chemistry unit that the high-energy phosphate bond of ATP is often written as a squiggle (~) to help us remember it’s a “special high-energy” bond, not just your average straight-line (covalent) bond.
Several different organ systems work together to maintain energy homeostasis. That’s what it means to be an organism, after all.
Media Attributions
- U02-065 and U2-066 and U05-005 Energy Profile of a Chemical Reaction © Hutchins, Jim is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U05-006 metabolism graphic © Hutchins, Jim is licensed under a Public Domain license
- Catabolism,_energy_carriers_and_anabolism © Muessig is licensed under a CC BY-SA (Attribution ShareAlike) license
- U05-007 Unit 5 burning vs glycolysis New2 © Hutchins, Jim is licensed under a Public Domain license

