Electrons as the Source of Chemical Properties
Objective 2.4
2.4.1 Illustrate how the number and distribution of electrons is related to the chemical properties of an element.
Tom Cech, who was Dr. Hutchins’ teacher at the University of Colorado, won the Nobel Prize in 1989. He said:

Because those collisions involve the relatively large electron clouds, and not the relatively small and internal nuclei, it is the interaction of electrons with the collision of atoms that results in chemistry.
Electrons are arranged in energy levels called orbitals, and are paired together by their up or down spin. We use orbital diagrams or orbital notation to indicate the energy levels of all the electrons in an atom of a given element.
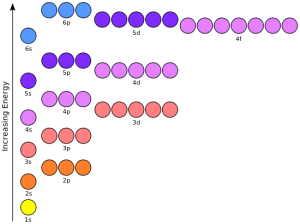
Each of these energy levels or orbitals wants to be completely filled. Once the orbital is filled, the electron cloud is in its lowest energy state (represented by a lower level on the diagram).
Aufbau is a German word that means “building up”. For this reason, the rule for filling orbitals is called the Aufbau Principle. Just like a multi-story building starts with a foundation, we fill the lowest energy orbital (the 1s) first; then the next level up (2s); then the next (2p); and so on. In the orbital diagrams shown here, the lowest energy level is lowest on the diagram.
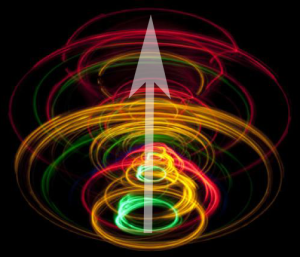
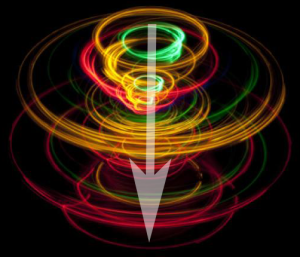
Electrons have spin. Remember we said chemistry is a metaphor? This is a picture which illustrates what no human has ever “seen”, which is the spin states of the electron. In orbital diagrams, spin states are represented as an “up arrow” or “down arrow”.
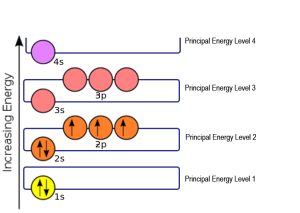 An atom of an element achieves its lowest energy state (most favorable configuration) by placing its electrons in a way that fills all its orbitals from the lowest to the highest energy. The example shown here is nitrogen, atomic number 7. Nitrogen has 7 protons (by definition) so a neutral nitrogen atom has 7 electrons: there are 7 packets of positive charge and 7 packets of negative charge for a net charge of zero. This orbital diagram shows the arrangement of the 7 electrons in nitrogen. The first orbital we fill with two electrons (one up, one down) is the 1s orbital. The second orbital we fill with two electrons is the 2s orbital. Now we’ve used 4 of our 7 alloted electrons and we have 3 left over. The up electron spins always go in first, and the 2p orbital has 3 parking spots to put them in, so we indicate 3 up electrons in those three circles.
An atom of an element achieves its lowest energy state (most favorable configuration) by placing its electrons in a way that fills all its orbitals from the lowest to the highest energy. The example shown here is nitrogen, atomic number 7. Nitrogen has 7 protons (by definition) so a neutral nitrogen atom has 7 electrons: there are 7 packets of positive charge and 7 packets of negative charge for a net charge of zero. This orbital diagram shows the arrangement of the 7 electrons in nitrogen. The first orbital we fill with two electrons (one up, one down) is the 1s orbital. The second orbital we fill with two electrons is the 2s orbital. Now we’ve used 4 of our 7 alloted electrons and we have 3 left over. The up electron spins always go in first, and the 2p orbital has 3 parking spots to put them in, so we indicate 3 up electrons in those three circles.
If this nitrogen atom adds three electrons with “down” spin, its 2p orbital will be filled and it will be in the lowest possible energy state. Since electrons have a -1 charge, we say nitrogen has a valence of -3 (3 x -1 = -3).
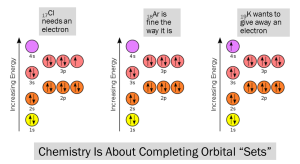
Like filling a parking garage from the bottom floors up, we want to fill the “parking spaces” in each “floor” or orbital for maximum efficiency. Chemistry is about completing those orbital sets. So if we have one open “parking spot” we want to find an electron to fill it, and if we have one car taking up an entire floor we want to give it away to get the most efficient “parking configuration”. In real terms, an atom has less energy if the entire set of orbitals (1s, 2s, 2p, 3s, etc.) is filled.
Sodium, chlorine, and many other atoms are rarely found in the body as uncharged atoms. Rather, in water, the sodium chloride (NaCl, table salt) molecule breaks into two charged atoms: Na+ and Cl–. The number of protons doesn’t change because they are buried in the nucleus; the number of electrons changes, resulting in a cation or anion.
11Na has 11 protons and prefers to have 10 electrons:
+11 – 10 = +1
17Cl has 17 protons and prefers to have 18 electrons:
+17 – 18 = –1
Cations and anions in the blood are called electrolytes (English: “electricity” + Greek: “released”) because they carry electrical charge.
When atoms like oxygen, sulfur, selenium, fluorine, chlorine, or iodine are present as ions (either in a compound or in solution) we refer to them as oxide, sulfide, selenide, fluoride, chloride, or iodide. For example, NaCl is called sodium chloride.
Media Attributions
- U02-021a tom cech quote © Cech, Tom adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-022 Orbital representation diagram © CK-12 Foundation is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-023 spin up © Creativity103 adapted by Jordan West is licensed under a CC BY (Attribution) license
- U02-024 spin down © Creativity103 adapted by Jordan West is licensed under a CC BY (Attribution) license
- U02-025a Principal Energy Levels Nitrogen © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-026 completing orbitals © CK-12 Foundation adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license

