Diffusion
Objective 2.9
2.9.1 Explain how molecules diffuse. Illustrate diffusion with examples from biological systems.
2.9.2 Discuss how diffusion is related to increasing entropy (the Second Law of Thermodynamics).
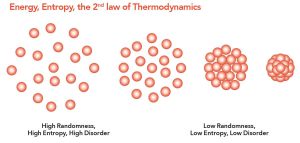
As particles move, they bounce around and distribute themselves all over. This is one aspect of a property called entropy: things become more disorganized in our houses. If we want to decrease entropy (i.e., increase organization) we have to do work—we have to expend energy to clean the house. The idea that disorder or randomness (formally, entropy) is increasing is called The Second Law of Thermodynamics.
This means that molecules in a liquid or gas, which are bouncing off each other, will eventually distribute themselves all over the container (i.e., increase the entropy of the closed system in the beaker). When we put ink in water, it eventually moves throughout the water and does not stay in one place. This is called diffusion. If there is no barrier, substances (like ink) always move from where they are at high concentration to where they are at low concentration. Given time, socks in your house will go from where they’re at high concentration (the sock drawer) to where they are at lower concentration (everywhere else).
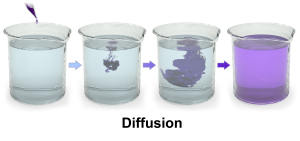
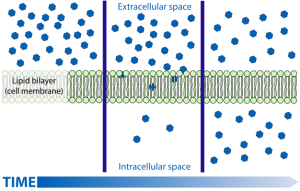
Understanding diffusion is essential for much of Unit 4, so take time now to make sure you are familiar with the concept. To take one specific example, diffusion moves molecules of the hormones 17β-estradiol (one of the estrogens) and dihydrotestosterone (one of the androgens) from the blood into the cells of the body, where these hormones can exert their effects on the DNA of the cell, permanently altering the cell’s fate. This is a large part of understanding the endocrine system in Unit 14.
A final principle of thermodynamics that we will use many times in the course is that energy is always transferred from where it is high to where it is low. That is, we can heat a pot of water by putting it on the stove, but we can’t cool it down by putting it on a “reverse stove”. Similarly, there’s no “reverse microwave” to cool down the baby formula you accidentally overheated. You can increase the number of collisions between the molecules that make up milk, but you can’t directly slow them down.
(If we put the baby bottle in a refrigerator or ice bath, the heat is transferred from the hot milk through the walls of the container into the cooler material. Similarly, a refrigerator works by heating your house, transferring heat from the inside of the refrigerator to the air in the kitchen. An air conditioner then cools your house by heating up the atmosphere around your house.)
Media Attributions
- U02-061 Diffusion © BruceBlaus is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-062 simple_diffusion_in_cell_membrane-en.svg © LadyofHats, Villareal, Mariana Ruiz is licensed under a Public Domain license
- U02-063 Biro-2nd-Law © Biró, Tamás Dr is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

