Chemical Equations
Objective 2.15
2.15.1 Begin to recognize how to symbolize chemical reactions.
2.15.2 Recognize when a chemical equation is balanced or unbalanced.
2.15.3 Compare dehydration synthesis to hydrolysis.
2.15.4 Explain why a chemical reaction is reversible.
To close out our study of chemistry, we’ll illustrate a few simple chemical reactions with chemical equations, a symbolic language that describes how chemicals react together.
Chemicals are all around us. (Scientists laugh when a product is labeled “chemical-free”; there is no such thing. Even pure water is full of the chemical compound H2O.) It shouldn’t surprise us then to see that we carry out chemical reactions every day, as illustrated in this section.
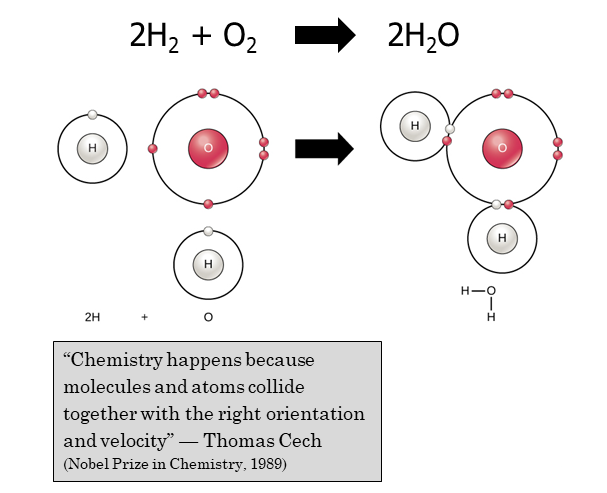
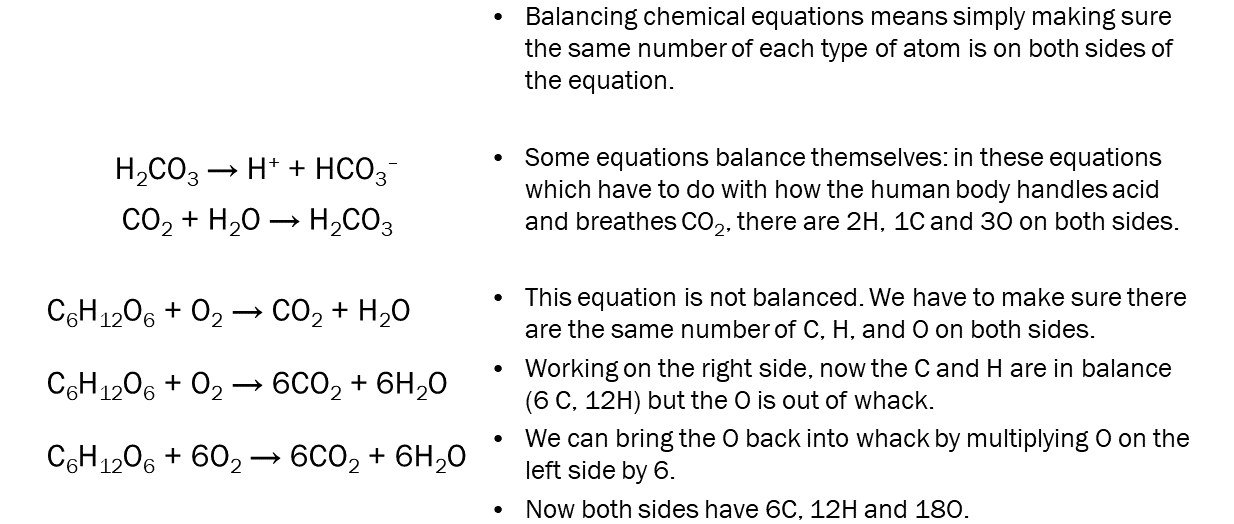
It’s important to chemists that chemical equations are balanced, which means the same number of atoms appear on both sides. For example, we can’t say H2 reacts with O2 to make H2O this way
H2 + O2 → H2O
because the reactants (left side) have 2 hydrogen atoms and 2 oxygen atoms, and the product (right side) has 2 hydrogen atoms and 1 oxygen atom.
We can fix this by adding some numbers
2H2 + O2 → 2H2O
Now the chemical equation is said to be balanced: each side has 4 hydrogens and 2 oxygens. The fancy word we use to describe the number of each reactant and product we get in a balanced chemical equation is stoichiometry.
This image shows the steps in balancing a chemical equation.
Some interesting chemical reactions, that will likely become more familiar to you during this course, are shown here.
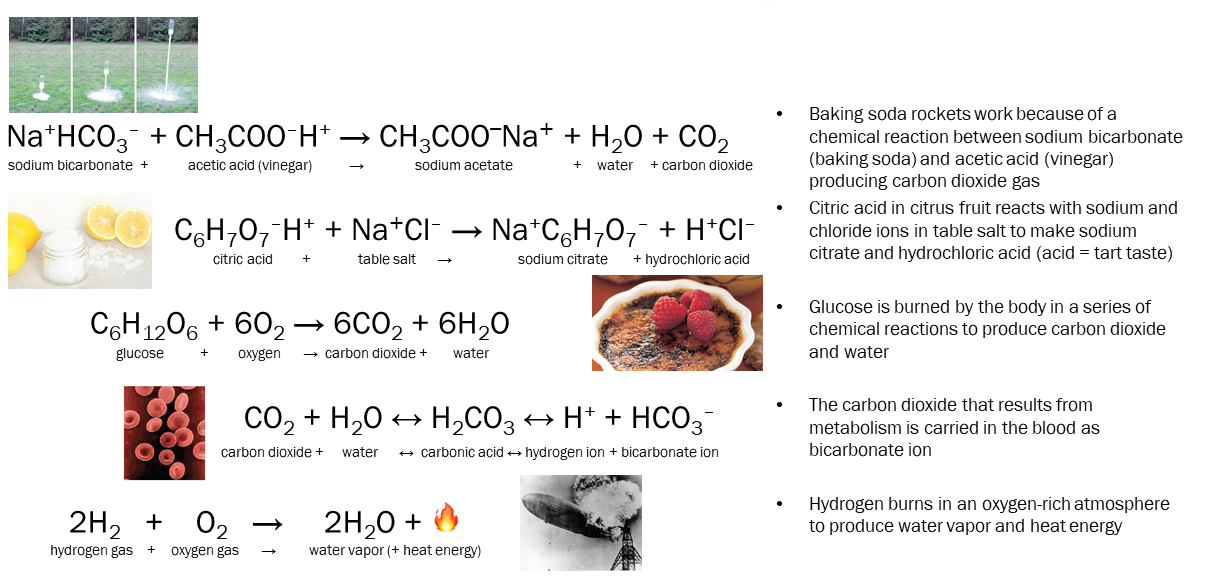
Note that when we symbolize chemicals, we follow the same order as on the periodic table: hydrogen, in group (column) 1, goes on the left and oxygen, in group (column) 16, goes on the right. When we have a polyatomic ion, we keep those intact: earlier in Objective 12, we met HCl, H2SO4, HNO3, NaHCO3, and NaOH which all follow these rules.
In the case of organic molecules, synthesis reactions are used to build (synthesize) larger compounds, and degradation reactions are used to break down (degrade) larger compounds into smaller ones.
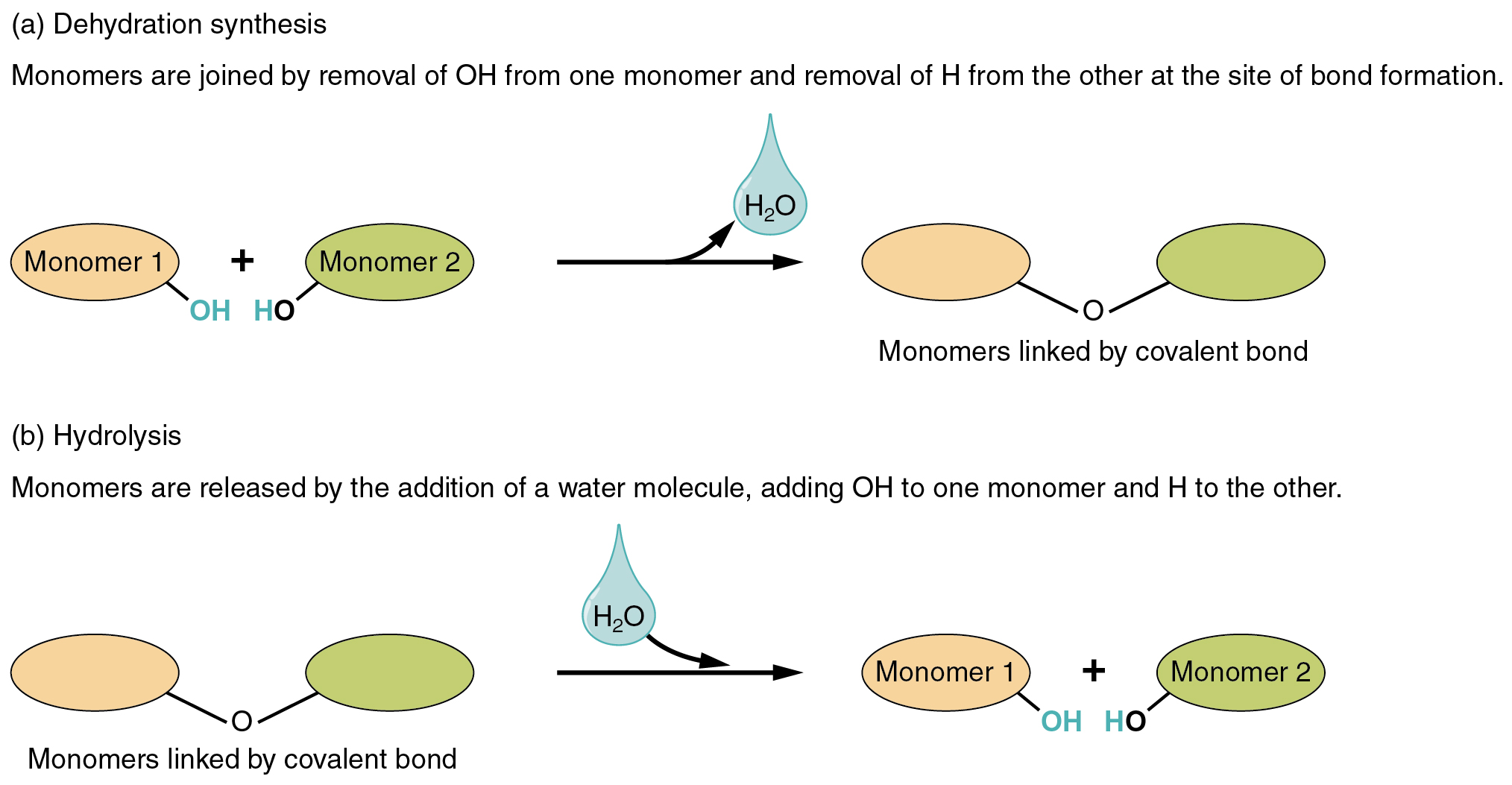
By far the most common type of synthesis reaction combines a hydroxyl (–OH) group from one small molecule and a hydrogen atom (–H) from another, removing water (HOH or H2O) in the process.
This dehydration synthesis is used to join many different monomers (small identical or similar molecules) into a polymer (a large molecule made up of smaller subunits). Deoxyribonucleic acid, which can reach sizes of 249 million smaller molecules strung together (human chromosome 1), is a good example of how we can take smaller molecules of only four types (A, C, G, and T) and assemble a huge molecule by dehydration synthesis.
To break up the larger polymer into smaller pieces, then, our cells reverse the process, called hydrolysis (“water breaking”). Since water was removed to make bigger molecules, it stands to reason that water is added to make smaller molecules. Water (HOH) contributes a –OH group to one small molecule and a –H to another and breaks the existing bond in the process.
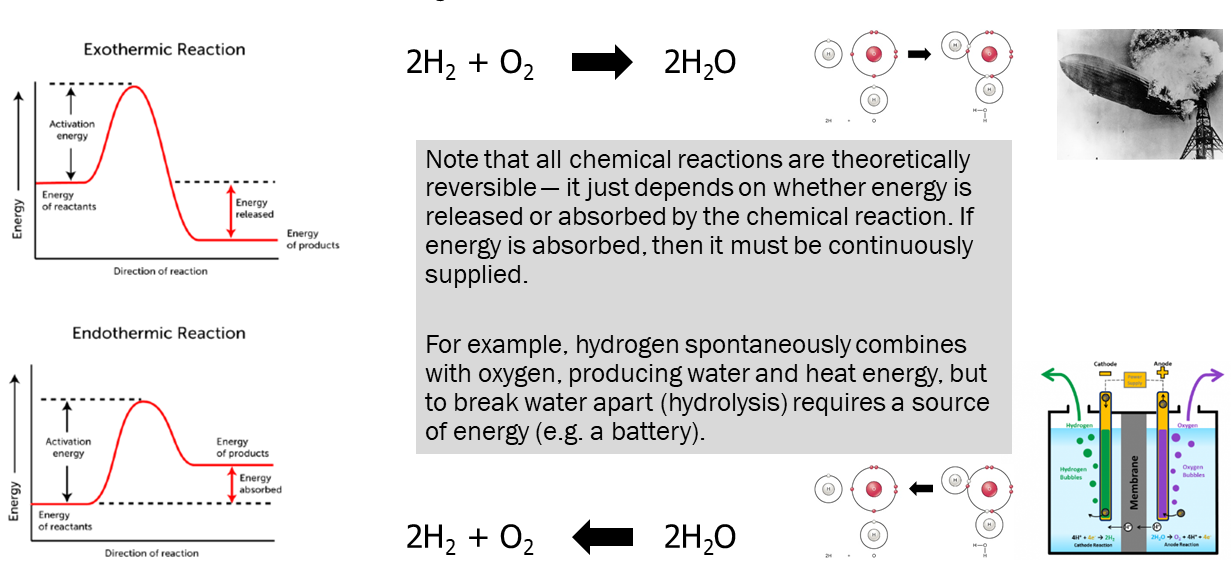
This illustrates a final, general principle: the reversibility of chemical reactions. Reactions can be exothermic (energy producing) or endothermic (energy absorbing) but there is nothing stopping a chemical reaction from reversing. If the reaction is endothermic, then the only requirement is a continuous supply of energy.
Exothermic reactions are, in general, spontaneous. When hydrogen gas (H2) burns in an oxygen (O2)-rich atmosphere, water molecules and energy are created. We start the reaction with a source of energy that gets a few molecules over the activation energy barrier. As they form product, there is energy left over. The energy goes into increasing the energy of the reactants, and makes it more likely that more H2 and O2 molecules are going to collide with the right orientation and velocity (i.e. achieve the activation energy needed to carry out the reaction). You should recognize this as a positive feedback loop like those we discussed in Unit 1.
On the other hand, the reverse reaction — the splitting apart of water into H2 and O2 — does not happen spontaneously. That is because the potential energy in the product (H2O) is higher than the sum of the potential energy in H-H and O=O. This energy has to be supplied. The only way we can get this reaction (electrolysis) to take place is by supplying energy, for example from a battery.
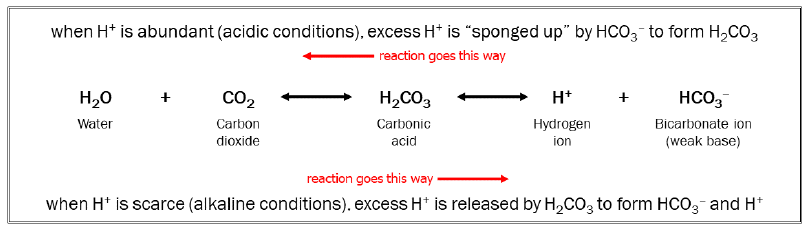
Some reactions have about the same amount of energy in all arrangements of atoms. The carbon dioxide/carbonic acid/bicarbonate system mentioned earlier is one of those. We make this reaction move to the right (as shown) by increasing the amount of CO2 available. (This is called the principle of mass action.) We make this reaction move to the left by increasing the amount of H+ available. In this way, CO2 and acid are interchangeable in the human body: a patient with too much CO2 in the blood is said to be in acidosis.
Media Attributions
- U02-103 hydrogen oxygen water © OpenStax is licensed under a CC BY (Attribution) license
- U02-104 chemical equations balanced © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-105 chemical equations © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-106 Dehydration_Synthesis_and_Hydrolysis-01 © OpenStax is licensed under a CC BY (Attribution) license
- U02-107 reversibility © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-108 bicarbonate graphic © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
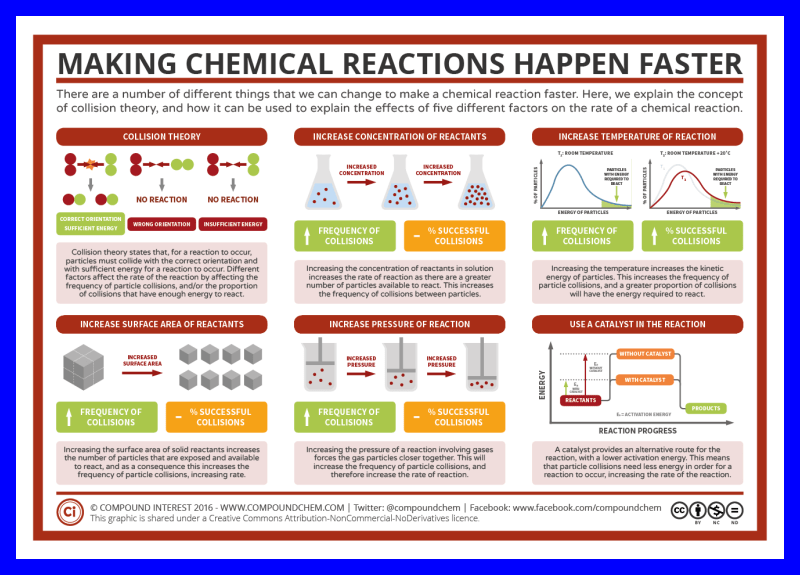
- U02-109 blue box © Andy Brunning is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

