Carbohydrates | Monosaccharides
Objective 3.7
3.7.1 Classify carbohydrates as pentoses or hexoses.
3.7.2 Recognize examples of each.
Next, we’ll look in detail at carbohydrates (sugars). Carbohydrate names end in –ose which makes it easy to identify these molecules from their chemical names.
The ratio of carbon to hydrogen to oxygen in carbohydrates is usually 1:2:1. Remember that water is H2O (2 hydrogen atoms bound to one oxygen atom). So, a carbohydrate always has the same number of carbon atoms as water molecules (for example, five carbons plus five waters would be C5H10O5). Indeed, the name “carbohydrate” means “watered carbon”.
Carbohydrates such as glucose often form ring structures with themselves. We see them drawn both ways: as a string of carbons with oxygens, hydrogens, and hydroxyl (–OH) groups attached; and as a ring. The molecule often rearranges to form this ring structure, as shown.
While carbohydrates can have between 3 and 7 carbons, the carbohydrates that we’ll study in this course either have five or six carbons. Five-carbon sugars are called pentoses while six-carbon sugars are called hexoses.
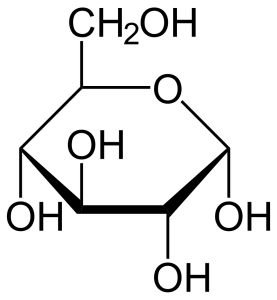
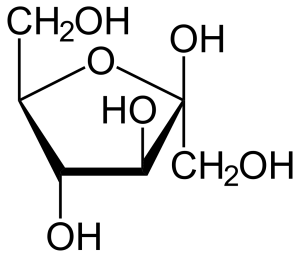
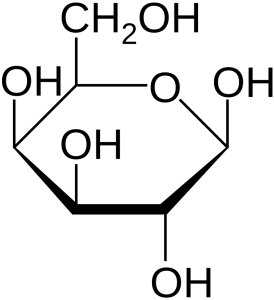
We said earlier that all carbohydrates have the formula CxH2xOx. That gives only two possible formulas for carbohydrates that we study in this course: pentoses (x=5) are C5H10O5 and hexoses (x=6) are C6H12O6. How can this be? The answer is, the carbon, hydrogen and oxygen atoms are arranged in different configurations and that makes different sugars. For example, glucose, fructose and galactose are all isomers of C6H12O6 but the atoms are arranged differently in each sugar. Count the atoms in each structural formula below to confirm this. Remember that in this shorthand form, an empty line means a hydrogen. Two, three, or four lines converging on a single point mean a carbon lives there. The remaining two, one, or zero spaces are occupied by hydrogen, to a total of four, since carbon always takes four bonds.
Molecules with the same number of atoms in different arrangements are called isomers.
Carbon-containing compounds exist in many isomers: the same number of carbon, nitrogen, oxygen, sulfur, and hydrogen atoms can make thousands of different compounds. For example, both glucose and fructose, two different kinds of sugar, have the formula C6H12O6.
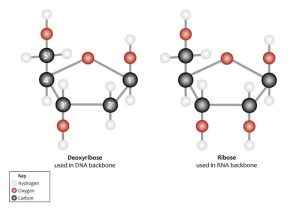
Hexoses are a food source. Pentoses are not. Rather, they are essential for the structure of nucleic acids, RNA and DNA. The sugar component of RNA is ribose. The sugar component of DNA is deoxyribose. Note the difference at position 2’ (2-prime) in the stick diagram. Ribose has an –OH (hydroxyl) group at that position whereas deoxyribose has just an –H. The lack of an oxygen is what gives deoxyribose its name.
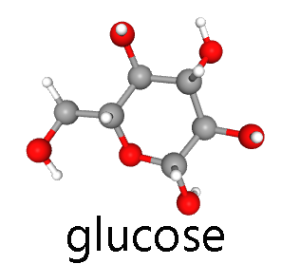
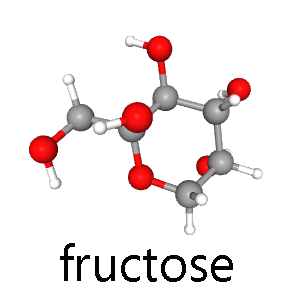
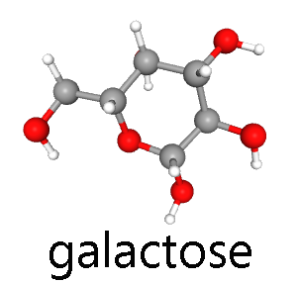
The three major hexoses, glucose, fructose, and galactose, are all found in significant quantities in the human diet.
 |
 |
 |
 |
 |
 |
Isomers are molecules comprised of the same atoms, but in different arrangements. All the hexoses (molecular formula C6H12O6) are isomers of each other.
Glucose is, by far, the most important of the hexoses. Human cells prefer glucose as an energy source. For this reason, glucose is the only significant component of “blood sugar” and we use the terms “blood sugar” and “blood glucose” interchangeably.
Fructose is a major sugar found in fruit and honey. In the body, fructose is converted to glucose for use by cells.
Galactose is a monosaccharide found as a component of lactose, or “milk sugar”. Lactose is a disaccharide composed of glucose and galactose.
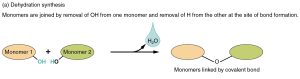
These monosaccharides listed above can join by dehydration synthesis. When two or more carbohydrate molecules are strung together this way, the series of dehydrations results in a carbohydrate polymer.
One sugar molecule = monosaccharide
Two sugar molecules joined by dehydration synthesis = disaccharide
Three or more sugar molecules joined by dehydration synthesis = polysaccharide
Media Attributions
- U03-077 U03-078 Chemical_structure_of_Ribose_and_Deoxyribose_(13080698355) modified with numbers © Genomics Education Programme is licensed under a CC BY (Attribution) license
- U03-081 U03-082 glucose 2d line drawing © NEUROtiker is licensed under a Public Domain license
- U03-083 U03-084 fructose 2d line drawing © NEUROtiker is licensed under a Public Domain license
- U03-085 U03-086 galactose 2d line drawing © NEUROtiker is licensed under a Public Domain license
- U03-081 U03-082 D-Glucose_Conformer3D_large (1) © Hutchins, Jim is licensed under a Public Domain license
- U03-083 U03-084 D-Fructose_Conformer3D_large © Hutchins, Jim is licensed under a Public Domain license
- U03-085 U03-086 D-Galactose_Conformer3D_large © Hutchins, Jim is licensed under a Public Domain license
- U03-011 same as U02-106 Dehydration_Synthesis_and_Hydrolysis-01 © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by J.D. Speth is licensed under a CC BY-SA (Attribution ShareAlike) license

