Atomic Number and Atomic Mass in the Periodic Table
Objective 2.7
2.7.1 Given a block of the periodic table, identify the atomic number and describe its significance.
2.7.2 Identify the atomic mass and describe how it is derived.
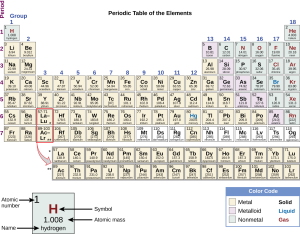 The atomic number is the number of protons. Remember that in a neutral atom, the number of protons and electrons is equal, but the electrons come and go, so the proton number is a reliable indicator of the chemical properties of that element.
The atomic number is the number of protons. Remember that in a neutral atom, the number of protons and electrons is equal, but the electrons come and go, so the proton number is a reliable indicator of the chemical properties of that element.
Mass number is the sum of protons and neutrons. Electrons are so light that they do not contribute to the mass number and are ignored.
The atomic mass (or atomic weight) is the average mass of all naturally occurring combinations of protons and neutrons in the nucleus. That is, the atomic mass depends on what isotopes of the element are found in nature.
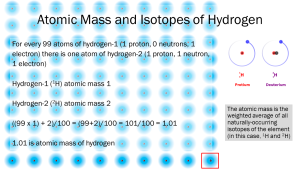
If we use an atomic butterfly net (not yet invented, but we can imagine it) and capture 100 atoms of hydrogen, we find that one out of 100 H atoms in nature is hydrogen 2, deuterium (2H). This is how we calculate the atomic mass (or atomic weight) that is shown in the periodic table. If 99 atoms are 1H, with a mass of 1 Da each, and 1 atom is 2H, with a mass of 2 Da, then the total mass of the 100 hydrogen atoms is 101 (99+2) and the average mass is 1.01 Da. If the math freaks you out, notice there are 100 hydrogen atoms in the figure above. Count the number of large particles (red protons and purple neutrons) and divide by 100 to get the average mass.
Carbon exists as 12C and 13C. There are about 99 12Cs for every one 13C, so its atomic mass is 12.01. Nitrogen exists as 14N or 15N, and there are about 99 14Ns for every 15N. That gives an atomic mass of 14.01.
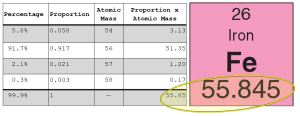
The most common isotopes of iron are 54Fe, 56Fe, 57Fe and 58Fe. What do the numbers 54, 56, 57, and 58 represent? How does that compare to 26, the atomic number for iron? How many neutrons are present in each case? (Hint: 54 massive particles are found in the nucleus of 54Fe, and the number of protons is always 26, so the number of neutrons is 54 – 26 = 28.)
Wikipedia says that the proportions of iron isotopes are 5.8% of 54Fe, 91.7% of 56Fe, 2.1% of 57Fe and 0.3% of 58Fe. Given this information, you can check and see if it agrees with the block of the periodic table. Since the most common isotope by far is 56Fe, we expect to get a number close to 56, and we do.
By rounding the atomic mass, we can easily determine the most common isotope of the element, with a couple of exceptions that you will not be tested on. The atomic mass of hydrogen is 1.01, and the most common isotope of hydrogen is 1H, as we’ve already explained.
The atomic mass of carbon is 12.01, and the most common isotope of carbon is 12C. The atomic mass of nitrogen is 14.01, and the most common isotope of nitrogen is 14N. The atomic mass of iron is 55.85, and the most common isotope of iron is 56Fe.
Media Attributions
- U02-028 periodic table © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. is licensed under a CC BY (Attribution) license
- U02-055 atomic mass and isotopes of hydrogen © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-056 atomic mass and isotopes of iron © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license

