Amino Acids
Objective 3.2
3.2.1 Recognize the structure of amino acids.
3.2.2 Define –R group.
3.2.3 Given a diagram of the molecule, recognize the following categories of amino acid –R groups: acidic, basic, non-polar, or polar uncharged.
Proteins are molecules which carry out many of the cell functions. All cells in your body have the same DNA, and mostly the same RNA, but they all have different proteins which reflect their function. For example, red blood cells are essentially bags of the protein hemoglobin and nothing else. Some white blood cells make proteins called immunoglobulins which help protect the body. Digestive organs like the stomach and pancreas make enzymes, proteins that help break down the molecules in food so your body can absorb them. Skin cells are bags of a protein called keratin. All these cells are different from one another because they make different proteins, even though they all contain the exact same DNA molecules.
The monomer which makes up proteins is an amino acid. There are 20 amino acids which are commonly encountered; these are listed below.
Small strings of amino acids are generally called polypeptides. There is no sharp dividing line between a polypeptide and the larger, longer, protein.
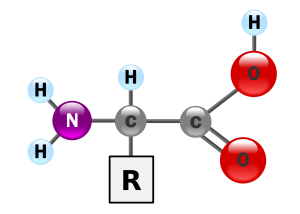
Amino acids have a simple structure: two carbons and a nitrogen form the backbone.
 |
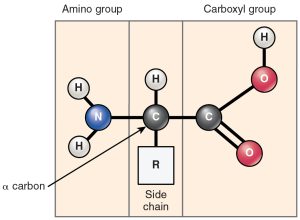 |
The nitrogen is bonded to two hydrogens in what is termed an amino group (–NH2) which gives the amino acids half of their name. The nitrogen is joined to a carbon, which is bonded to a variable group called “R”. R groups can be simple or complex. The last carbon is part of a carboxylic acid (carboxyl) group that gives the amino acid the second half of its name. A shorthand way to write the carboxyl group is –COOH.
Amino acids are joined together by peptide bonds. The formation of a peptide bond is another type of dehydration synthesis reaction.
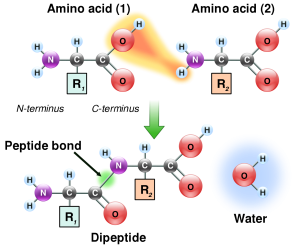
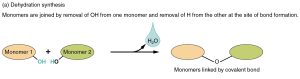
The –COOH of one amino acid and the –NH2 of another can react in a type of dehydration synthesis. Removing two hydrogens and one oxygen to make water, a peptide bond results and binds the nitrogen to a carbon. In this way, we can string together amino acids connected in sequence by a series of peptide bonds. The repeating –N–C–C– forms the backbone of a protein, and the –R groups protruding from the middle carbon (C) give the protein its characteristic folding pattern and chemical properties.
The resulting combination of two amino acids is called a dipeptide. More than two amino acids is called a polypeptide.
There is no clear dividing line for when a polypeptide is large enough to be termed a protein, but anything larger than 3000 to 10,000 Daltons molecular weight (that is, about 30 to 100 amino acids) would be called a protein by most scientists. Proteins can be quite large; the structural proteins of the body tend to be between 200,000 and 300,000 Daltons (200 to 300 kDa) in size, or about 2000 to 3000 amino acids.
Proteins are synthesized in a specialized cell machine called a ribosome that we will examine in Unit 6.
Enzymes called peptidases or proteases break peptide bonds by hydrolysis (recall that hydrolysis is the opposite of dehydration synthesis).
There are 20 amino acids that are commonly found in the human body. Amino acids are designated by a three-letter or single-letter abbreviation. The Canvas course has a link to tables which show both abbreviations; you don’t need to learn either set but it’s good to know how to find the information should you need it.
The –R group is shown in a blue shaded box in the figures shown here. Note the different atoms of each –R group, giving each amino acid a unique set of properties.
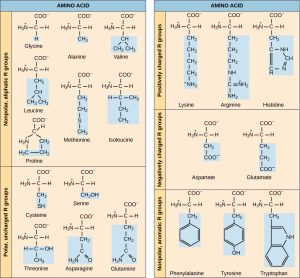
Each of the amino acids has some unique property or feature to lend to the structure and function of a protein, and to the function of the human body.
For example, the –R groups of aspartic acid and glutamic acid each have a carboxyl (–COOH) group. As the names imply, these –R groups have an acidic nature.
Remember that an acid acts as a hydrogen ion donor, so these carboxylic acid (–COOH) groups can donate a hydrogen and become a charged carboxyl (–COO–) –R group. The electronegative oxygens have kept hydrogen’s electron (e–) and donated a proton (H+).
When that happens, we formally change the name to aspartate and glutamate, as shown in the diagram. In practice, biochemists tend to use these terms interchangeably: glutamic acid = glutamate and aspartic acid = aspartate.
Lysine, arginine, and histidine are basic amino acids. Remember from Unit 2 that bases are hydrogen ion acceptors, so they carry a positive charge due to the presence of an extra H+.
Ten of the 20 amino acids are non-polar, and prefer a waterless environment; they group together.
Remember that polar covalent bonds, which occur because of the moderate difference in electronegativity between C and O, H and O, or H and N (Unit 2, Objective 5).
These –R groups are hydrophobic because the atoms in them (C, H, S) have similar electronegativity and so form non-polar covalent bonds. These non-polar –R groups will associate closely with the lipids in the cell membrane (Objective 12).
You will notice that methionine is part of this amino acid grouping. Methionine is the first amino acid added to make every protein.
Here, O and N are associated with ring (aromatic) chemical groupings. (These are called “aromatic” for the exact reason you’d expect: they’re smelly chemicals.) It’s beyond this course to explain why, but aromatic structures tend to “cool off” the electronegativity difference between O or N and carbon, and so these are still classified as non-polar. Tyrosine and tryptophan still have some chemical reactivity and are used as the basis for neurotransmitters and hormones, as we’ll see in Units 13 and 14.3-19
The last group of five amino acids shown here is polar uncharged, which means they associate with water (i.e. are hydrophilic) but are neither acidic nor basic. These hydrophilic –R groups tend to have hydroxyl or amino groups which form polar covalent bonds within the –R group and hydrogen bonds between the –R groups or between these –R groups and water.
A few other unique properties of -R groups are worth mentioning since you will see many them in later units.
Asparagine is found in asparagus, from which it gets its name.
Glutamate and glycine are also used as signaling molecules between cells.
Tyrosine and tryptophan are used as precursors for the so-called biogenic amine transmitters, which we will study in the nervous system units.
Histidine is made into histamine, which is an inflammatory signal in the immune system (Unit 15).
Glycine is the simplest amino acid, with an –R group consisting of a single hydrogen atom, and is really neither polar nor non-polar. Some authorities classify it one way, and some the other.
Tyrosine’s carbon ring structure allows an iodine to be added, which is the basis for thyroid hormone molecules.
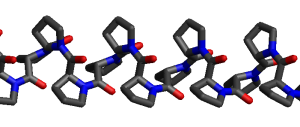
The unusual shape of proline puts hairpin turns (“kinks”) in a protein’s structure. Polyproline (a molecule consisting of a chain of prolines joined by peptide bonds) is a very kinky polypeptide indeed, as you can see from this figure.
Media Attributions
- U03-018 monomer polymer definition table (p3-14A) © Weber State University Department of Health Sciences is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-019 amino acid © YassineMrabet is licensed under a Public Domain license
- U03-020 amino acid structure © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U03-024 peptide bond © YassineMrabet is licensed under a Public Domain license
- U03-011 same as U02-106 Dehydration_Synthesis_and_Hydrolysis-01 © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by J.D. Speth is licensed under a CC BY-SA (Attribution ShareAlike) license
- U03-027 amino acid structures © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- U03-033 Poly_Pro_I_sideview © WillowW is licensed under a Public Domain license

