Acids, Bases, and the Concept of pH
Objective 2.12
2.12.1 Define: acid and base.
2.12.2 Describe how acids donate hydrogen ions.
2.12.3 Explain the formation of hydronium ions.
2.12.4 Describe how bases accept hydrogen ions.
2.12.5 Summarize what makes an acid or base strong or weak.
2.12.6 Describe the concept of pH.
2.12.7 Given a pH value, be able to state whether it is acidic, neutral, or basic.
2.12.8 Understand the reasons why a biological fluid might be acidic or basic.
2.12.9 Discuss how buffers moderate changes in pH.
Substances like HCl share characteristics of polar covalent and ionic compounds. There is a significant probability, approaching 100% or 1.0, that H+Cl– will split apart into H+ and Cl– ions. Similarly, there is a near-100% probability that H2SO4 will split into H+ and HSO4– and that HNO3 will split into H+ and NO3–.
Therefore, all these compounds are called acids. You may already know these acids by name:
- HCl is hydrochloric acid (used to clean concrete or add chlorine ions to swimming pools)
- H2SO4 is sulfuric acid (used in lead-acid car batteries)
- HNO3 is nitric acid
A chemist named Lewis then decided that defining an acid was nice and easy: according to Lewis, acids are hydrogen ion donors.
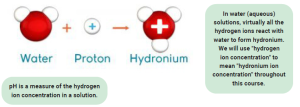
 But, as Tina Turner said, “we never do anything nice and easy”. Also, Lewis’ definition leaves a lot to be desired. Therefore, two chemists named Brønsted and Lowry tried a different definition, and that’s the one that is more useful in biochemistry. The Brønsted-Lowry definition of an acid starts with the donation of a H+ but doesn’t end there. H+ then reacts with H2O to form hydronium ion (H3O+):
But, as Tina Turner said, “we never do anything nice and easy”. Also, Lewis’ definition leaves a lot to be desired. Therefore, two chemists named Brønsted and Lowry tried a different definition, and that’s the one that is more useful in biochemistry. The Brønsted-Lowry definition of an acid starts with the donation of a H+ but doesn’t end there. H+ then reacts with H2O to form hydronium ion (H3O+):
H+ + H2O → H3O+

In the Brønsted-Lowry conception of bases, a base is a hydrogen ion acceptor. Bases have a full or partial extra electron which attracts and holds a positively-charged hydrogen ion (proton). Bases are also called alkalis and basic solutions are called alkaline.
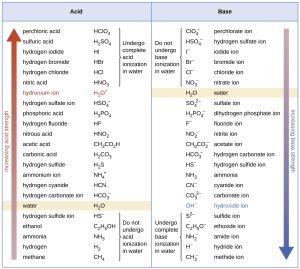
A twist in the acid-base story is that acids and bases can be strong or weak.
You know this already. Only a cruel person would offer to put battery acid (H2SO4) on your tongue, but a chef might offer to put citric acid (C6H8O7) on your tongue. (Citric acid is the acid that gives citrus fruits like lemons their tang.) H2SO4 is a strong acid. Citric acid is a weak acid.

Similarly, you might drink baking soda (NaHCO3) in water to settle a sour stomach, but you probably wouldn’t want to drink the drain cleaner lye (NaOH) to settle your stomach. Severe tissue damage would result if you did. NaHCO3 is a weak base. NaOH is a strong base.

So if acids are hydrogen ion donors, what makes them strong or weak? Strong acids like HCl completely fall apart in water to donate all their hydrogen ions, while weak acids like citric acid only donate 1 H+ for every 1408 molecules of citric acid.
Similarly, strong bases like NaOH accept all the hydrogen ions they can find, while only 1 in 4700 NaHCO3 molecules accept a hydrogen ion.
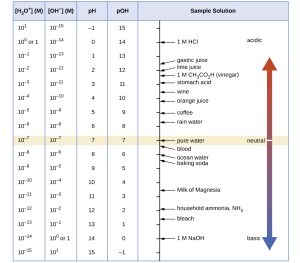
We use a quantity called the pH to define how strong or weak an acid or base is. The pH scale runs from 0 to 14. The pH is formally defined as “the negative logarithm of the hydrogen ion concentration” but for our purposes, we don’t need to learn the formal definition. We do need to know which pH values represent acids and bases, and how the scale works. Do not memorize these values; rather use them to understand the pH scale.
Strong acids completely dissociate in water to release all their hydrogen ions. Solutions of strong acids have a pH near zero.
Weak acids only partly dissociate in water. Lime juice, a solution of citric acid, has a pH of 2. Orange juice is even less acidic than lime juice, with a pH of about 4.
About 1 in 100,000,000,000,000 water molecules spontaneously break apart into H+ and OH– ions. If you care to do the math (we assume you don’t) and apply the definition of pH, that means that pure water has a pH of 7. A pH of 7 is called neutral and it is the only value that is neither acid nor base.
Blood is a weak base. It has a pH of 7.4, just slightly above 7. Baking soda in water has a pH of 8.3, more basic than blood. Bleach (sodium hypochlorite) has a pH of 12.6, which is quite basic, and lye (NaOH) is the most basic substance we commonly encounter, with a pH of 14. That means all the NaOH molecules in water break apart into Na+ and OH– ions.
Weak acids are used in the body to kill bacteria, viruses, and fungi. Stomach acid (pH about 2-3) not only kills bacteria but helps break down swallowed food into small molecules, because hydrogen ions participate in a lot of chemical reactions that our digestive system finds useful. Vaginal fluid (pH 3.8-4.5) is mildly acidic, which helps maintain the health of this organ. 2-65
Canned tomatoes must have a pH below 6, according to USDA home canning guidelines; this helps inhibit the growth of deadly Clostridium botulinum, the bacterium that causes botulism. It’s prudent to test the pH of your home-canned tomatoes with pH papers.

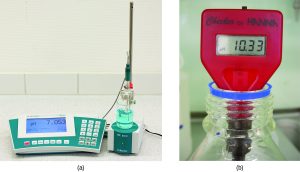
In the lab, it’s more common to use a pH meter to measure the pH of a solution.
When we place a strong acid such as H+Cl– into water, the H+Cl– dissociates into H+ and Cl–. The resulting H+ then reacts with water (H2O) to form hydronium ion (H3O+), meeting our definition of acid.
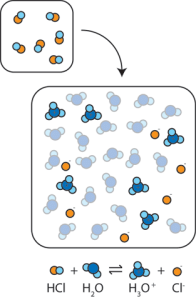
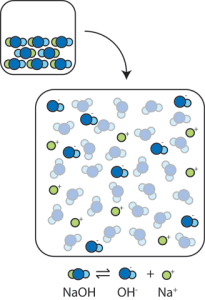
When we place a strong base such as the ionic compound Na+OH– into water, it dissociates into Na+ and OH– ions. The OH– ions act as H+ acceptors; the reaction between H+ and OH– forms water:
H+ + OH– → HOH (H2O)
Thus, OH– meets our definition of base.
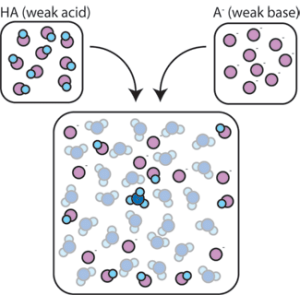 Weak acids and weak bases are important components of a special type of solution called a buffer. A buffer is a substance which acts as a “sponge” to hold and inactivate H+ and/or OH–. In this way, it stabilizes the concentration of H+ and/or OH–, which in turn stabilizes the pH. This figure shows the creation of a buffer by mixing a weak acid and a weak base.
Weak acids and weak bases are important components of a special type of solution called a buffer. A buffer is a substance which acts as a “sponge” to hold and inactivate H+ and/or OH–. In this way, it stabilizes the concentration of H+ and/or OH–, which in turn stabilizes the pH. This figure shows the creation of a buffer by mixing a weak acid and a weak base.
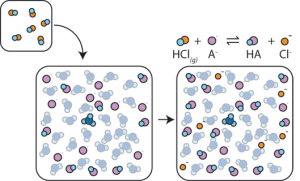 Now when we add acid to this buffer mixture, the H+ ions associate with the weak base anion (A–) to form the weak acid HA. This keeps the H+ ions from reacting with water to make hydronium (H3O+). The less hydronium we make, the less the pH changes.
Now when we add acid to this buffer mixture, the H+ ions associate with the weak base anion (A–) to form the weak acid HA. This keeps the H+ ions from reacting with water to make hydronium (H3O+). The less hydronium we make, the less the pH changes.
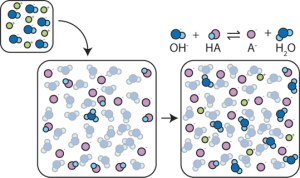 And when we add base to this buffer mixture, the weak acid HA donates its H+ to the OH– ions we added, making water and the weak base A–. The more hydrogen acceptor OH– we remove in this way, the less the pH changes.
And when we add base to this buffer mixture, the weak acid HA donates its H+ to the OH– ions we added, making water and the weak base A–. The more hydrogen acceptor OH– we remove in this way, the less the pH changes.
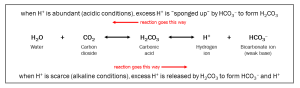
The most important biological buffer is the system of carbon dioxide, carbonic acid, and bicarbonate (CO2/H2CO3/HCO3–). Note that when CO2 is dissolved in water (H2O), carbonic acid is formed:
H2O + CO2 ↔ H2CO3 ↔ H+ + HCO3–
Each of the double-headed arrows is a step where H+ can be donated or accepted. Note that all parts of this complicated chemical equation have 2 H, 1 C and 3 O so this is just a rearrangement of the same atoms.
The pH of blood is carefully maintained by homeostatic mechanisms to lie between 7.35 and 7.45, and in healthy individuals is almost always exactly 7.40. This pH is kept constant by the carbonic acid—bicarbonate buffer system mentioned earlier. The lungs and kidneys work together to maintain the pH of blood as close to 7.40 as possible.
Media Attributions
- U02-075 hydronium ion with graphics © Alchetron adapted by Jordan West is licensed under a CC BY-SA (Attribution ShareAlike) license
- U02-076 Hydronium © Bensaccount is licensed under a Public Domain license
- CNX_Chem_14_01_conjugate_img © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. adapted by Jordan West is licensed under a CC BY (Attribution) license
- U02-077a strong acid graphic © Hutchins, Jim
- U02-077b strong bases graphic © Hutchins, Jim
- U02-078 strength of conjugate acid-base pairs © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. is licensed under a CC BY (Attribution) license
- U02-079 classification of acid base neutral solutions © Flowers, Paul; Theopold, Klaus; Langley, Richard; Austin, Stephen F.; Neth, Edward J. is licensed under a CC BY (Attribution) license
- U02-080 pH indicators © OpenStax is licensed under a CC BY (Attribution) license
- U02-081 pH meter © OpenStax is licensed under a CC BY (Attribution) license
- U02-082 HCl creates H3O © Chemcollective is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-083 NaOH creates OH © Chemcollective is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-083a buffer before © Chemcollective is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-084 buffer plus acid © Chemcollective is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-085 buffer plus base © Chemcollective is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-086 bicarb © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license

