States of Matter
Objective 2.8
2.8.1 Name the three states of matter found in the human body, and describe characteristics associated with each.
2.8.2 Describe the relationship of molecules to one another in a solid, liquid, or gas.
All biological materials exist in one of three states: gas, liquid, or solid.
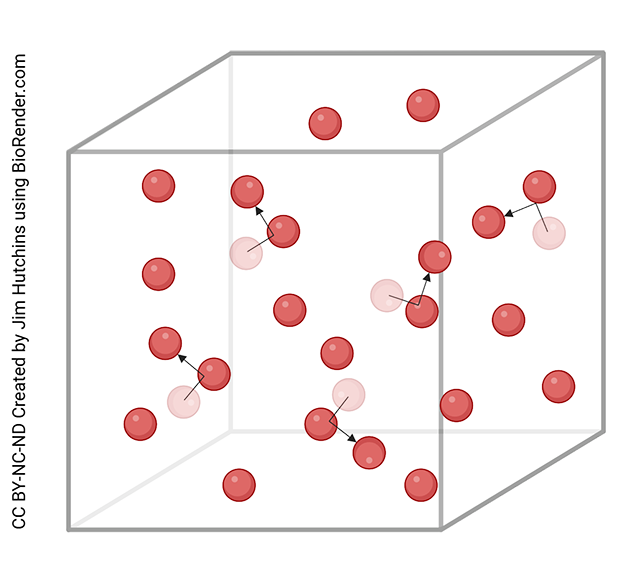 Gases have widely spaced particles that are flying around, banging off of each other. The particles don’t interact much beyond these collisions. This gives gases their special properties we’ll discuss later. Gases have an indefinite shape and indefinite volume. That is, gases are very compressible.
Gases have widely spaced particles that are flying around, banging off of each other. The particles don’t interact much beyond these collisions. This gives gases their special properties we’ll discuss later. Gases have an indefinite shape and indefinite volume. That is, gases are very compressible.
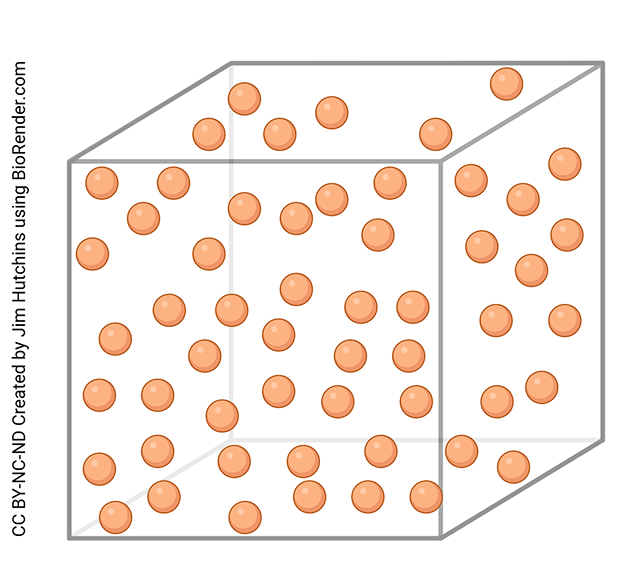 Liquids contain particles that are interacting with one another, but much more weakly. A liquid changes its shape to match the container it’s in, so it’s said to have an indefinite shape. However, the volume is definite, and liquids are not very compressible. At the submicroscopic level, liquids contain particles that are colliding with each other but even during those collisions they maintain some sort of interaction with each other.
Liquids contain particles that are interacting with one another, but much more weakly. A liquid changes its shape to match the container it’s in, so it’s said to have an indefinite shape. However, the volume is definite, and liquids are not very compressible. At the submicroscopic level, liquids contain particles that are colliding with each other but even during those collisions they maintain some sort of interaction with each other.
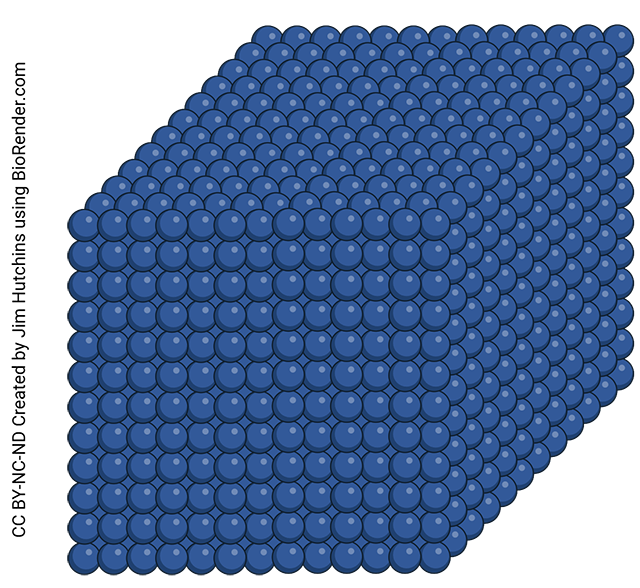 Solids are substances in which the particles are tightly associated with each other. Solids have a definite shape and definite volume and are not very compressible (which is why we sit on solid substances and not other kinds).
Solids are substances in which the particles are tightly associated with each other. Solids have a definite shape and definite volume and are not very compressible (which is why we sit on solid substances and not other kinds).
The same molecules make up solids, liquids, and gases. They change states depending on the relationship between the molecules. That relationship depends on temperature and pressure. As pressure increases, the relationship between molecules becomes closer and closer until they are in a rigid array. This conception of how molecules are related to each other in the three forms of matter is formally called the kinetic molecular theory.
For water, this rigid array the solid form, is called ice. In solid ice, water molecules are vibrating, but they do not move past each other. That means that ice maintains its shape and you can stand on it, for example when ice fishing.
Temperature is a way of describing the motion of particles. The higher the temperature, the more molecular motion that takes place. The more molecular motion that takes place, the higher the temperature. The lowest possible temperature in the universe, called absolute zero, is the temperature at which there is no molecular motion at all. Since there can’t be any less motion than nothing, absolute zero is truly absolute zero. This happens at –273.15°C which is also described as 0 Kelvin or 0 K. The Kelvin scale is the same as the Celsius scale, it just starts at –273.15°. Thus, the surface temperature of the human body is 37°C or 310 K.
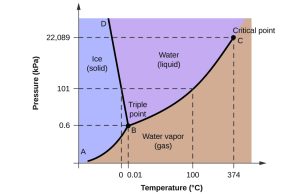
You will want to spend some time practicing how to interpret this graph.
Follow the dotted line which indicates atmospheric pressure (101 kPa) on the phase diagram. At 0°C, water transitions from the solid form to the liquid form. The molecules of water are more loosely related to each other, forming temporary bonds that break and reform easily. Because the molecules can slide past each other, they no longer adopt a defined shape but fill the container they’re put into.
At 100°C, water transitions from the liquid form to the gaseous form (water vapor). Now the molecules of water are bouncing off each other like little billiard balls on speed. There is no relationship between the water molecules except for when they collide with each other (about 10 billion times per second!). Understanding the relationship of molecules of O2, N2, Ar, and CO2 in the atmosphere is essential for our understanding of how the respiratory system works, which we’ll address in Unit 17.
Understanding that billions of molecular collisions per second occur even in liquids is fundamental for an understanding of our next concept, diffusion.
Media Attributions
- U02-057 states of matter gas © Hutchins, Jim is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-058 states of matter liquid © Hutchins, Jim is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-059 states of matter solid © Hutchins, Jim is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U02-060 water phase diagram © LibreTexts is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license

