Scott Crousillac
Unit Outline
Learning Objectives
At the end of this unit, you should be able to:
I. Describe the “fluid mosaic” model of membrane structure.
II. Describe how the structure of the cell membrane affects membrane permeability.
III. Describe the following passive transport processes: diffusion, facilitated diffusion, and osmosis.
IV. Describe the effects of placing red blood cells in hypertonic, hypotonic, and isotonic solutions, respectively.
V. Describe the following active transport processes: primary and secondary active transport, endocytosis (phagocytosis, pinocytosis, receptor-mediated endocytosis), and exocytosis
Part 1: The Cell Membrane
Despite differences in structure and function, all living cells in multicellular organisms have a surrounding cell membrane. As the outer layer of your skin separates your body from its environment, the cell membrane (also known as the plasma membrane) separates the inner contents of a cell from its exterior environment. This cell membrane provides a protective barrier around the cell and regulates which materials can pass in or out.
Structure and Composition of the Cell Membrane
The cell (plasma) membrane is described by the fluid mosaic model; it is an extremely pliable structure composed primarily of stacked phospholipids (a “bilayer”). Cholesterol is also present, which contributes to the fluidity of the membrane, and there are various proteins embedded within the membrane that have a variety of functions.
A single phospholipid molecule has a phosphate group on one end, called the “head,” and two side-by-side chains of fatty acids that make up the lipid tails (Figure 1). The phosphate group is negatively charged, making the head polar and hydrophilic—or “water loving.” A hydrophilic molecule (or region of a molecule) is one that is attracted to water and is water soluble. The phosphate heads are thus attracted to the water molecules of both the extracellular and intracellular environments. The lipid tails, on the other hand, are uncharged, or nonpolar, and are hydrophobic, or “water fearing,” and are NOT water soluble. A hydrophobic molecule (or region of a molecule) repels water. An amphipathic molecule is one that contains both a hydrophilic and a hydrophobic region. In fact, soap works to remove oil and grease stains because it has amphipathic properties. The hydrophilic portion can dissolve in water, while the hydrophobic portion can trap grease in micelles that then can be washed away.
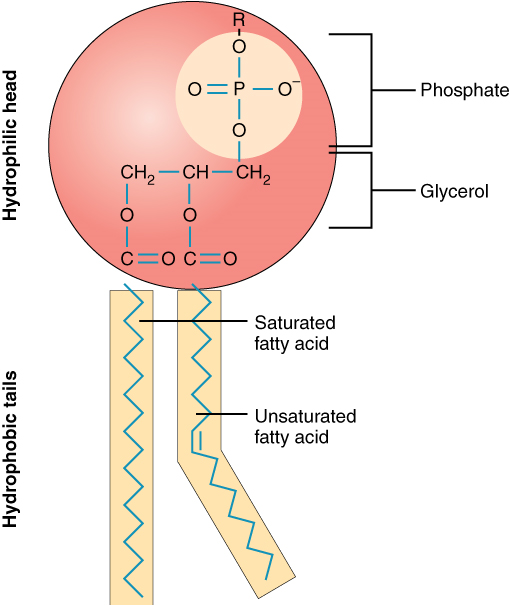
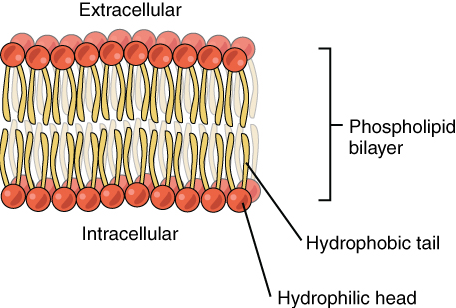
The cell membrane consists of two adjacent layers of phospholipids. The lipid tails of one layer face the lipid tails of the other layer, meeting at the interface of the two layers. The phospholipid heads face outward, one layer exposed to the interior of the cell and one layer exposed to the exterior (Figure 2). Because the phosphate groups are polar and hydrophilic, they are attracted to water in the intracellular fluid. Intracellular fluid (ICF) is the fluid in the interior of the cell. The phosphate groups are also attracted to the extracellular fluid. Extracellular fluid (ECF) is the fluid environment outside the enclosure of the cell membrane. Interstitial fluid (IF) is the term given to extracellular fluid not contained within blood vessels. Because the lipid tails are hydrophobic, they meet in the inner region of the membrane, repelling watery intracellular and extracellular fluid from this space.
The cell membrane has many proteins, as well as other lipids (such as cholesterol), that are associated with the phospholipid bilayer. An important feature of the membrane is that it remains relatively fluid; the lipids and proteins in the cell membrane are not rigidly locked in place but can move, the way that molecules are able to move about freely in a liquid. Thus, the plasma membrane behaves more like a liquid than it does a solid. This feature explains the “fluid” component of the fluid mosaic model.
Membrane Proteins
The lipid bilayer forms the basis of the cell membrane, but it is populated throughout with various proteins, representing the “mosaic” part of the fluid mosaic model. Two different types of proteins that are commonly associated with the cell membrane are the integral proteins and peripheral proteins (Figure 3). As its name suggests, an integral protein is a protein that is embedded in the membrane. Integral proteins that span the entire plasma membrane are referred to as transmembrane proteins. A channel protein is an example of an integral protein that selectively allows particular materials, such as certain ions, to pass into or out of the cell. Channel proteins are crucial for cell function because charged particles such as ions would not be able to freely cross the plasma membrane.
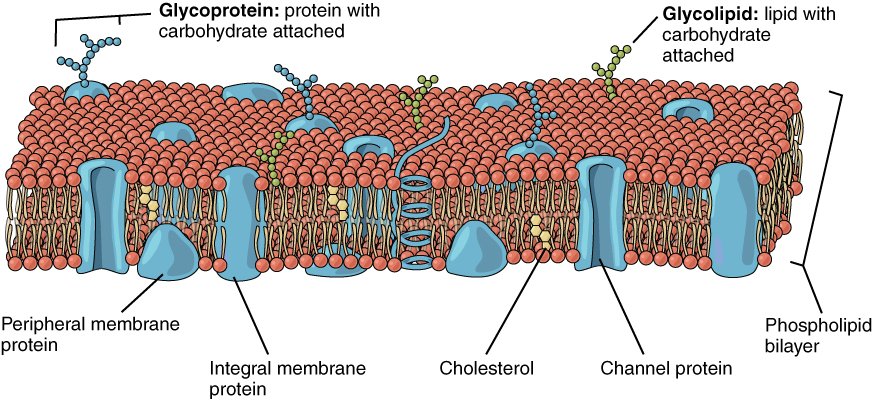
Some integral membrane proteins are glycoproteins. A glycoprotein is a protein that has a carbohydrate molecule attached, which extends into the extracellular matrix. The attached carbohydrates on glycoproteins aid in cell recognition. The glycocalyx is a fuzzy-appearing coating around the cell formed from glycoproteins and other carbohydrates attached to the cell membrane. The glycocalyx can have various roles. For example, it may have molecules that allow the cell to bind to another cell, it may contain receptors for hormones, or it might have enzymes to break down nutrients. The glycocalyces found in a person’s body are products of that person’s genetic makeup. They give each of the individual’s trillions of cells the “identity” of belonging to the person’s body. This identity is the primary method for a person’s immune defense cells to recognize and not attack the person’s own body cells, but it also is the reason organs donated by another person might be rejected.
Peripheral proteins are typically found on the inner or outer surface of the lipid bilayer but can also be attached to the internal or external surface of an integral protein. These proteins typically perform a specific function for the cell. Some peripheral proteins on the surface of intestinal cells, for example, act as digestive enzymes to break down nutrients to sizes that can pass through the cells and be absorbed into the bloodstream.
Test Your Knowledge
I. Describe the “fluid mosaic” model of the membrane structure including the membrane components.
- Describe the characteristics of the plasma membrane according to the fluid mosaic model (i.e., explain why it is appropriate to refer to the membrane as “fluid” AND why it is appropriate to refer to the membrane as a “mosaic.”)
II. Describe how the structure of the cell membrane affects membrane permeability.
- Describe how the structural components of the plasma membrane make it “selectively permeable” rather than permeable or impermeable. In your description, be sure to refer to the types of molecules that may pass easily (or not) through the membrane and what chemical characteristics they share that makes them capable (or incapable) of doing so.
Part 2: Transport across the Cell Membrane
One of the great wonders of the cell membrane is its ability to regulate the concentration of substances inside the cell. These substances include ions such as Ca2+, Na+, K+, and Cl−; nutrients including sugars, fatty acids, and amino acids; and waste products, particularly carbon dioxide (CO2), which must leave the cell.
The membrane’s lipid bilayer structure provides the first level of control. The phospholipids are tightly packed together, and the membrane has a hydrophobic interior. This structure causes the membrane to be selectively permeable. A membrane that has selective permeability allows only substances meeting certain criteria to pass through it unaided. In the case of the cell membrane, only relatively small, non-polar materials can move through the lipid bilayer (remember, the lipid tails of the membrane are nonpolar). Some examples of these are other lipids, oxygen and carbon dioxide gases, and alcohol. However, water-soluble materials—like glucose, some amino acids, and electrolytes—need some assistance to cross the membrane because they are repelled by the hydrophobic tails of the phospholipid bilayer. All substances that move through the membrane do so by one of two general methods, which are categorized based on whether or not energy is required. Passive transport is the movement of substances across the membrane using their own kinetic energy, without the expenditure of chemical energy. This is simply a matter of substances naturally diffusing from an area of high concentration to an area of low concentration. In contrast, active transport is the movement of substances across the membrane using some form of energy to move that substance from an area of low concentration to an area of high concentration. For primary active transport, this energy is supplied from the hydrolysis of Adenosine Triphosphate (ATP). For secondary active transport, the energy is provided by another substance moving down its chemical gradient.
Passive Transport
In order to understand how substances move passively across a cell membrane, it is necessary to understand concentration gradients and diffusion. A concentration gradient is the difference in concentration of a substance across a space. Molecules (or ions) will spread from where they are more concentrated to where they are less concentrated until they are equally distributed in that space. (When molecules move in this way, they are said to move down their concentration gradient.) Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. A couple of common examples will help to illustrate this concept. Imagine being inside a closed bathroom. If a bottle of perfume were sprayed, the scent molecules would naturally diffuse from the spot where they left the bottle to all corners of the bathroom, and this diffusion would go on until no more concentration gradient remains. Another example is a spoonful of sugar placed in a cup of tea. Eventually, the sugar will diffuse throughout the tea until no concentration gradient remains. In both cases, if the room is warmer or the tea hotter, diffusion occurs even faster, as the molecules are bumping into each other and spreading out faster than at cooler temperatures. Having an internal body temperature around 37.5° C thus also aids in the diffusion of particles within the body.
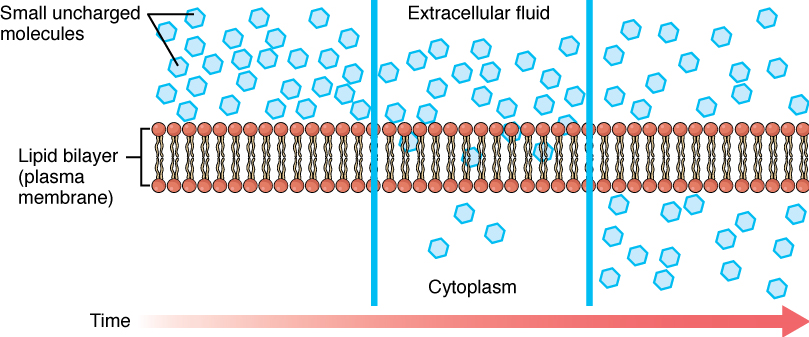
Whenever a substance exists in greater concentration on one side of a semi-permeable membrane than on the other side, such as the cell membranes, any substance that can move down its concentration gradient across the membrane will do so. Consider substances that can easily diffuse through the lipid bilayer of the cell membrane, such as the gases oxygen (O2) and CO2. O2 generally diffuses into cells because it is more concentrated outside of them; and CO2 typically diffuses out of cells because it is more concentrated inside of them. In both these examples, the molecules rely on their own kinetic energy to move, so neither of these examples requires any additional energy. The movement of molecules across a cell membrane without the expenditure of cellular energy is referred to as passive transport, or diffusion (Figure 4).
Before moving on, you need to review the gases that can diffuse across a cell membrane. Because cells rapidly use up oxygen during metabolism, there is typically a lower concentration of O2 inside the cell than outside. As a result, oxygen will diffuse from the interstitial fluid into the cytoplasm within the cell. On the other hand, because cells produce CO2 as a byproduct of metabolism, CO2 concentrations rise within the cytoplasm; therefore, CO2 will move from the cell into the interstitial fluid, where its concentration is lower. Both these molecules are small and non-polar, which means they can easily interact with the hydrophobic core of a lipid bilayer and move between the molecules to get from one side to the other. This mechanism of small, non-polar molecules slipping between the lipid tails of a cell membrane from the side where they are more concentrated to the side where they are less concentrated is a form of passive transport called Simple Diffusion (Figure 4).
Large polar or ionic molecules, which are hydrophilic, cannot easily cross the phospholipid bilayer. Charged atoms or molecules of any size cannot cross the cell membrane via simple diffusion, as the charges are repelled by the hydrophobic tails in the interior of the phospholipid bilayer. Solutes dissolved in water on either side of the cell membrane will tend to diffuse down their concentration gradients, but because most substances cannot pass freely through the lipid bilayer of the cell membrane, their movement is restricted to protein channels and specialized transport mechanisms in the membrane. Facilitated diffusion is the diffusion process used for those substances that cannot cross the lipid bilayer due to their size, charge, and/or polarity (Figure 5). A common example of facilitated diffusion is the movement of glucose into the cell. Although glucose can be more concentrated outside of a cell, it cannot cross the lipid bilayer via simple diffusion because it is both large and polar.
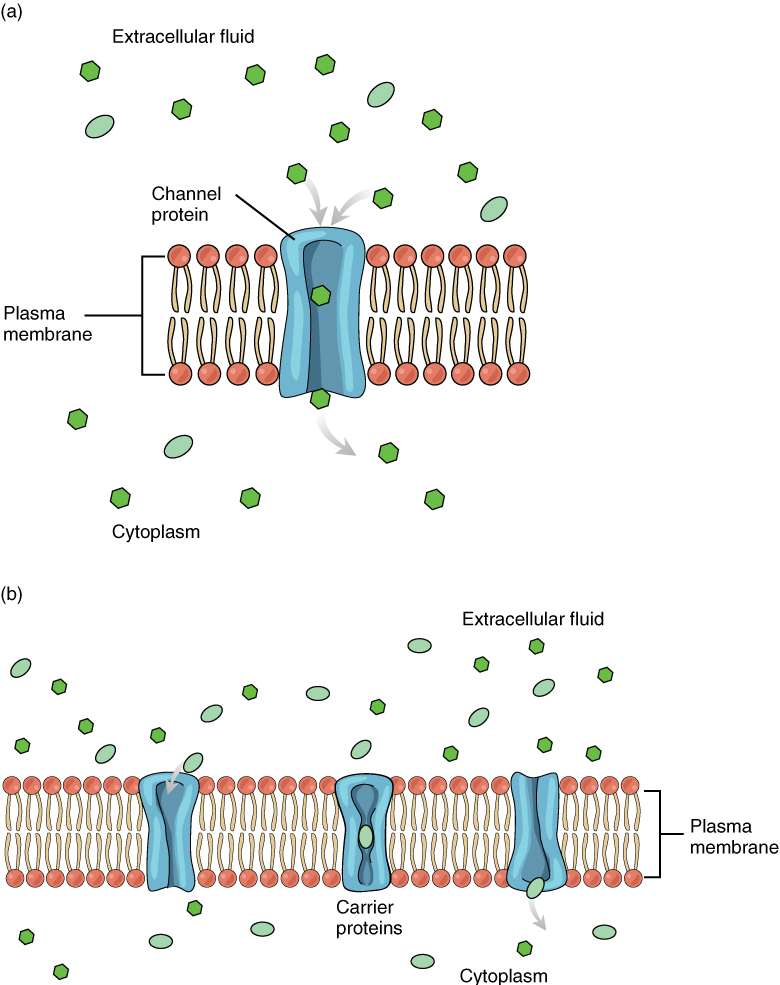
To resolve this, a specialized carrier protein called the glucose transporter will transfer glucose molecules into the cell. Glucose and other relatively large polar molecules typically bind to transport proteins that change shape to allow the molecules into the cell by a process known as carrier-mediated facilitated diffusion.
The use of a protein that acts as a channel through which an ion or small polar molecule can move down its concentration gradient is referred to as channel-mediated facilitated diffusion. For example, sodium ions (Na+) are highly concentrated outside of cells. These electrolytes are charged and cannot pass through the non-polar lipid bilayer of the membrane. Their diffusion is facilitated by membrane proteins that form sodium channels (or “pores”) so that Na+ ions can move down their concentration gradient from outside the cells to inside the cells.
There are many other solutes that must undergo facilitated diffusion to move into a cell, such as amino acids, or to move out of a cell, such as wastes. Because facilitated diffusion is a passive process, it does not require energy expenditure by the cell.
Very small, polar molecules, including water, can cross the phospholipid bilayer via simple diffusion due to their small size. The rate at which water can move across cell membranes is increased by the presence of membrane proteins called aquaporins that form channels through which water molecules (but not solutes) can pass. Osmosis refers to the passive movement of water across a semipermeable membrane (Figure 6). Osmosis across a cell membrane, therefore, includes the movement of water molecules by either simple diffusion or facilitated diffusion or both.
The movement of water across a cell membrane cannot be always easily regulated by cells, so it is important that cells are exposed to an environment in which the concentration of solutes outside of the cells (in the extracellular fluid) is equal to the concentration of solutes inside the cells (in the cytoplasm). Two solutions that have the same concentration of solutes are said to be isotonic (equal tension). When cells and their extracellular environments are isotonic, the concentration of water molecules is the same outside and inside the cells, and the cells maintain their normal shape (and function).
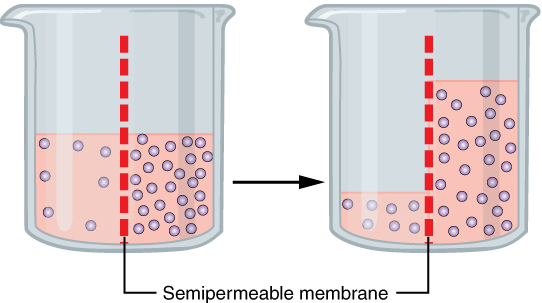
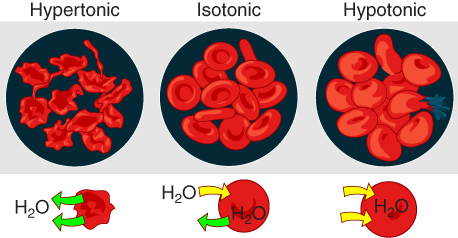
Osmosis occurs when there is an imbalance of solutes outside of a cell versus inside the cell. A solution that has a higher concentration of solutes than another solution is said to be hypertonic, and water molecules tend to diffuse into a hypertonic solution (Figure 7). Cells in a hypertonic solution will shrivel as water leaves the cell via osmosis. In contrast, a solution that has a lower concentration of solutes than another solution is said to be hypotonic, and water molecules tend to diffuse out of a hypotonic solution.
Cells in a hypotonic solution will take on water and swell, with the risk of eventually bursting. A critical aspect of homeostasis in living things is to create an internal environment in which all of the body’s cells are in an isotonic solution. Various organ systems, particularly the kidneys, work to maintain this internal environment.
Active Transport
For all of the transport methods described above, the cell does not need to use additional energy because substrates are moving down their concentration gradients (from high to low concentration). Membrane proteins that aid in the passive transport of substances do so without the hydrolysis of ATP. During primary active transport, the energy released from ATP hydrolysis is required to move a substance across a membrane, often with the help of carrier proteins and usually against the concentration gradient of the substance being moved.
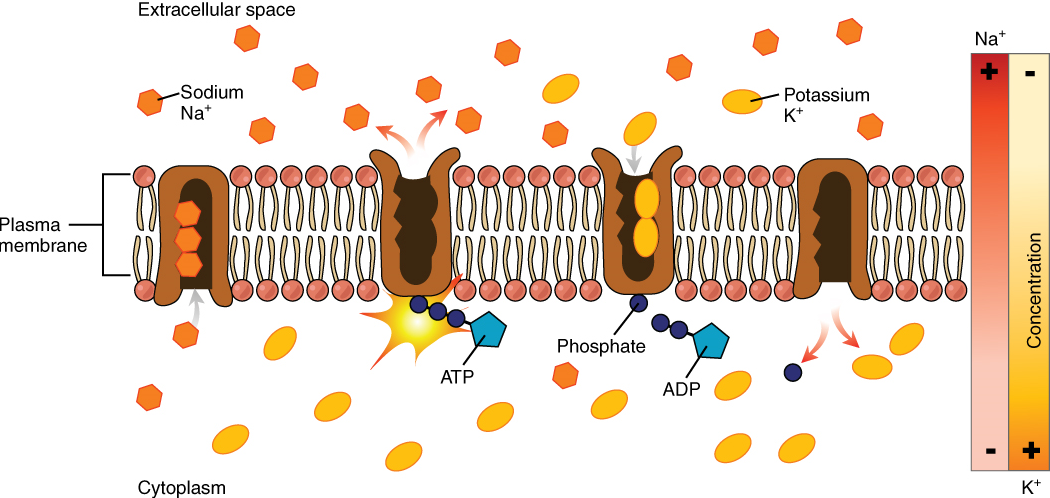
One of the most common types of primary active transport involves proteins that serve as pumps. The word “pump” in biological systems will always indicate that energy is required in order for the mechanism to function. Chemical energy from ATP is required for these membrane proteins to transport substances—molecules or ions—across the membrane, usually against their concentration gradients (from an area of low concentration to an area of high concentration).
The Sodium-Potassium Pump, which is also called Na+/K+ ATPase, transports sodium out of a cell while moving potassium into the cell, both against their gradients. The Na+/K+ pump is an important ion pump found in the membranes of many types of cells. These pumps are particularly abundant in neurons, which are constantly pumping out sodium ions and pumping in potassium ions to maintain an electrical gradient across their cell membranes. An electrical gradient is a difference in electrical charge across a space. In the case of neurons, for example, the electrical gradient exists between the inside and outside of the cell, with the inside being negatively charged relative to the outside. The negative electrical gradient is maintained because each Na+/K+ pump moves three Na+ ions out of the cell and two K+ ions into the cell for each ATP molecule that is hydrolyzed (Figure 8). This process is so important for neurons that it accounts for the majority of their ATP usage.
Active transport pumps can also work together with other active or passive transport systems to move substances across the membrane. For example, the sodium-potassium pump maintains a high concentration of sodium ions outside of the cell. Therefore, if the cell needs sodium ions, all it has to do is open a passive sodium channel, as the concentration gradient of the sodium ions will promote their diffusion into the cell. In this way, the action of an active transport pump (the sodium-potassium pump) powers the passive transport of sodium ions by creating a concentration gradient. When active transport of one substance is used to power the transport of another substance in this way, it is called secondary active transport, to distinguish it from primary active transport mechanisms that use the chemical energy released from ATP to directly drive the movement of an ion or molecule. The two main subcategories of secondary active transport are cotransport and countertransport.
Cotransport involves a scenario in which both substances are moving in the same direction. An example would be Na+ diffusing down its electrochemical gradient into the cell, providing the energy needed to move glucose into the cell as well.
Countertransport involves a scenario in which two substances are moving in opposite directions. An example would be Na+ diffusing down its electrochemical gradient into the cell, providing the energy needed to move H+ (protons) out of the cell.
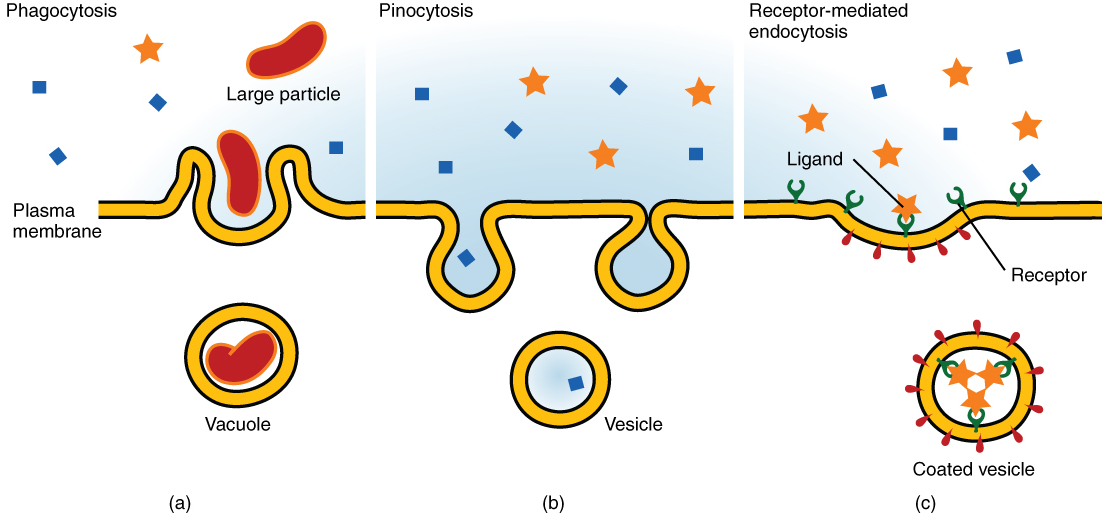
Other forms of active transport do not involve membrane carriers. Endocytosis (bringing “into the cell”) is the process of a cell ingesting material by enveloping it in a portion of its cell membrane and then pinching off that portion of membrane (Figure 9). Once pinched off, the portion of membrane and its contents becomes an independent, intracellular vesicle. A vesicle is a membranous sac—a spherical and hollow organelle bound by a lipid bilayer membrane. Endocytosis often brings materials into the cell that must be broken down or digested. Phagocytosis (“cell eating”) is the endocytosis of large, non-specific particles. Many immune cells engage in phagocytosis of invading pathogens. Their job is to patrol body tissues for foreign matter, such as invading bacterial cells, phagocytizing them, and digesting them. In a process similar to phagocytosis, pinocytosis (“cell drinking”) brings non-specific fluid containing dissolved substances into a cell through membrane vesicles.
Phagocytosis and pinocytosis take in large portions of extracellular material, and they are typically not highly selective in the substances they bring in. Cells regulate the endocytosis of specific substances via receptor-mediated endocytosis. Receptor-mediated endocytosis is endocytosis by a portion of the cell membrane that contains many receptors that are specific for a certain substance. Once the surface receptors have bound sufficient amounts of the specific substance, the cell will endocytose the part of the cell membrane containing the complex. Iron, a required component of hemoglobin, is endocytosed by red blood cells in this way. Iron is bound to a protein called transferrin in the blood. Specific transferrin receptors on red blood cell surfaces bind the iron-transferrin molecules, and the cell endocytoses the complex.
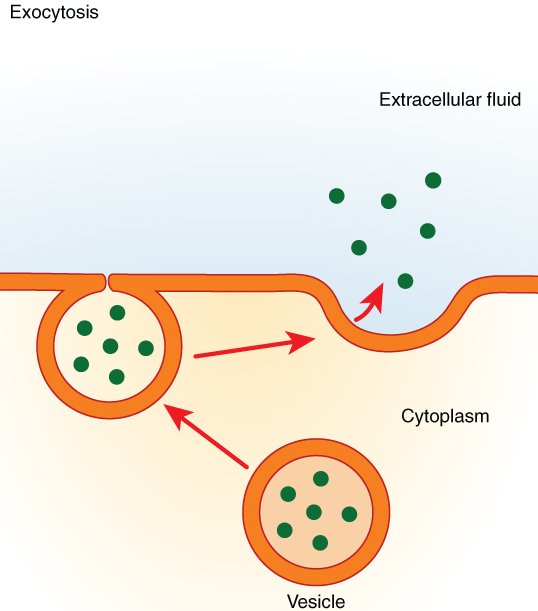
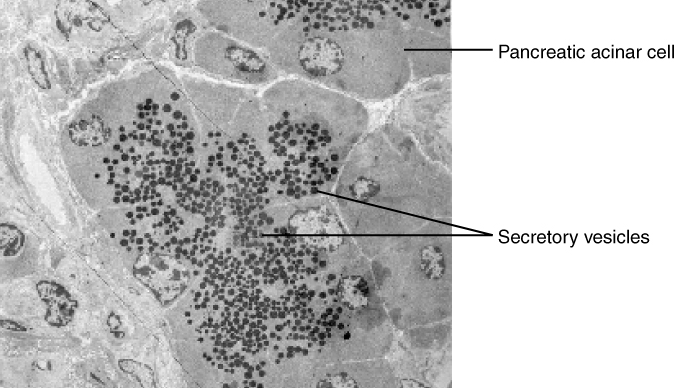
In contrast with endocytosis, exocytosis (taking “out of the cell”) is the process of a cell exporting material using vesicular transport (Figure 10). This process is essentially endocytosis in reverse. Many cells manufacture substances that must be secreted, like a factory manufacturing a product for export. These substances are typically packaged into membrane-bound vesicles within the cell. When the vesicle membrane fuses with the cell membrane, the vesicle releases its contents into the interstitial fluid. The vesicle membrane then becomes part of the cell membrane. Cells of the stomach and pancreas produce and secrete digestive enzymes through exocytosis (Figure 11). Endocrine cells produce and secrete hormones that are sent throughout the body, and certain immune cells produce and secrete large amounts of histamine, a chemical important for immune responses.
Test Your Knowledge
III. Describe the following passive transport processes: diffusion, facilitated diffusion, and osmosis. Explain the function of each in a cell.
IV. Describe and explain the effects of placing red blood cells in hypertonic, hypotonic, and isotonic solutions, respectively.
- Describe and explain the effects (i.e., on cell size, cell shape, and cytosol solute concentrations) of placing red blood cells in a solution that is:
- Hypertonic relative to the cytosol
- Hypotonic relative to the cytosol
- Isotonic relative to the cytosol
V. Describe the following active transport processes: primary and secondary active transport, endocytosis (phagocytosis, pinocytosis, receptor-mediated endocytosis), and exocytosis. Explain the function of each in a cell.
- Compare and contrast (with the use of annotated diagrams) the characteristics of the following in terms of (a) ATP requirements, (b) molecules moved, (c) size of material moved, and (d) the direction of movement (i.e., relative to its own concentration gradient, relative to another molecule’s concentration gradient, and/or relative to the cell’s internal vs. external environment):
- Active and passive transport mechanisms
- Simple and facilitated diffusion
- Facilitated diffusion and osmosis
- Primary and secondary active transport
- Facilitated diffusion and secondary active transport
- Exocytosis and endocytosis
- Pinocytosis and phagocytosis
- Phagocytosis and receptor-mediated endocytosis

Practice
For the exercise below, drag the answers to the correct empty boxes.
Image Description
Figure 7.3 image description: The plasma membrane is a phospholipid bilayer containing many different molecular components. Among these are glycoproteins (proteins with a carbohydrate attached), glycolipids (carbohydrates with lipids attached), peripheral proteins on both sides, integral proteins, cholesterol, and channel proteins. [Return to image.]
Figure 7.6 image description: In the beaker on the left, the solution on the right side of the membrane is hypertonic relative to the solution on the left side of the membrane. So, in order to reach equilibrium, water moves from left to right across the semi-permeable membrane. [Return to image.]
Figure 7.7 image description: The figure shows erythrocytes in solutions that are hypertonic, isotonic, or hypotonic relative to cytosol. In a hypertonic environment, erythrocytes will shrivel as water leaves the cells. In an isotonic environment, erythrocytes will not change, as water enters and exits the cells at the same rate. In a hypotonic environment, erythrocytes will swell as water moves into the cells. [Return to image.]
Figure 7.8 image description: In the figure, there is a higher concentration of sodium outside of the cell relative to inside. There is a higher concentration of potassium inside the cell relative to the outside. Because of this, sodium tends to diffuse into the cell, while potassium tends to diffuse out of the cell. Over time, without the sodium-potassium pump, these ion gradients would reach equilibrium, disrupting the electrical activity of the cell. In a single cycle of the sodium-potassium pump, three sodium ions are extruded from and two potassium ions are imported into the cell. [Return to image.]
Chemically, a type of steroid, cholesterol is a component of cell membranes and a precursor of some important vitamins and hormones.
Class of organic compounds that are composed of many amino acids linked together by peptide bonds.
“Water loving”; a molecule or portion thereof that is polar and therefore water soluble.
“Water hating”; a molecule or portion thereof that is nonpolar and therefore water insoluble.
Molecule that contains both hydrophilic and hydrophobic regions.
Fluid inside cells.
Fluid outside cells (plasma or interstitial fluid).
Extracellular fluid in the small spaces between cells not contained within blood vessels.
Membrane-associated protein that spans the entire width of the lipid bilayer.
Membrane proteins that span the entire width of the plasma membrane.
Protein that has one or more carbohydrates attached.
Coating of carbohydrate molecules that surrounds the cell membrane.
Membrane-associated protein that does not span the width of the lipid bilayer, but is attached peripherally to integral proteins, membrane lipids, or other components of the membrane.
Feature of any barrier that allows certain substances to cross but excludes others.
Form of transport across the cell membrane that does not require input of cellular energy.
Form of transport across the cell membrane that requires input of cellular energy.
Active transport using carrier proteins that use ATP (powered by the energy obtained through phosphorylation by ATP).
Chemical reaction in which a molecule water is split into H and OPH, thereby breaking a bond and severing a compound.
Nucleotide containing ribose and an adenine base that is essential in energy transfer.
Active transport using pumps (carrier proteins) that are powered by the potential energy of a concentration gradient (usually of H+ or Na+).
Difference in the concentration of a substance between two regions.
Movement of a substance from an area of higher concentration to one of lower concentration.
A form of passive transport across a cell membrane that does not require a membrane protein.
Diffusion of a substance with the aid of a membrane protein.
Facilitated diffusion mechanism that utilizes a carrier protein that changes shape during the transport process.
Facilitated diffusion mechanism that utilizes a channel protein that has a pore, which only allows certain substances to pass through.
Membrane-spanning protein that has an inner pore, which allows the passage of one or more substances (a form of facilitated diffusion).
Building block of proteins; characterized by an amino and carboxyl functional groups and a variable side-chain.
Diffusion of water molecules down their concentration gradient across a selectively permeable membrane.
Describes a solution concentration that is the same as a reference concentration.
Describes a solution concentration that is higher than a reference concentration.
Describes a solution concentration that is lower than a reference concentration.
Steady state of body systems that living organisms maintain.
Membrane-spanning protein that binds to substances it needs to transport, changes shape, and moves the substance into or out of the cell (a form of facilitated diffusion or active transport pumps when energy is required).
Primary active transport protein that uses ATP hydrolysis to pump 3 sodium ions out of the cell and 2 potassium ions into the cell.
A difference in electrical charge across a space.
Form of secondary active transport in which two substances are moving in the same direction, with one substance providing energy for the other.
Secondary active transport mechanism in which two substances are moving in opposite directions, with one substance providing energy to pump the other substance.
Import of material into the cell by formation of a membrane-bound vesicle.
Membrane-bound structure that contains materials within or outside of the cell.
Cell process (a form of endocytosis) in which a cell engulfs and ingests another large particle or cell.
Endocytosis of fluid.
Endocytosis of ligands attached to membrane-bound receptors.
Protein molecule that contains a binding site for another specific molecule (called a ligand).
Export of a substance out of a cell by formation of a membrane-bound vesicle.
Tissue or organ that secretes hormones into the blood and lymph without ducts such that they may be transported to organs distant from the site of secretion.
Secretion of an endocrine organ that travels via the bloodstream or lymphatics to induce a response in target cells or tissues in another part of the body.
Vasoactive (active on blood vessels) mediator in granules of mast cells and is the primary cause of allergies and anaphylactic shock.

