Scott Crousillac
Unit Outline
Part 1. Biological Importance of Water
- Water as a Lubricant and Cushion
- Water as a Heat Sink
- Water as a Component of Liquid Mixtures
- The Role of Water in Chemical Reactions
Learning Objectives
At the end of this unit, you should be able to:
I. Explain the biological importance of water.
II. Specify the percentage of body weight that is composed of water and estimate the amount of body water you contain in liters.
III. Describe the distribution of body water.
As much as 70% of a human’s body weight is water. This water is contained both within the cells and between the cells that make up tissues and organs. Its several roles make water indispensable to human function.
Part 1. Biological Importance of Water
Water as a Lubricant and Cushion
Water is a major component of many of the body’s lubricating fluids. Just as oil lubricates the hinges on a door, water in synovial fluid lubricates the actions of body joints, and water in pleural fluid helps the lungs expand and recoil during breathing. Watery fluids help keep food flowing through the digestive tract and ensure that the movement of adjacent abdominal organs is frictionless.
Water also protects cells and organs from physical trauma. Water is a major component of CSF (Cerebrospinal Fluid), which cushions the brain within the skull. Water also helps protect the delicate nerve tissue of the eyes and cushions a developing fetus in the mother’s womb.
Water as a Heat Sink
A heat sink is a substance or object that absorbs and dissipates heat but does not experience a large corresponding increase in temperature. Because of its ability to form numerous hydrogen bonds, water naturally resists large changes in temperature. Consider a pot of water that has been heated on a stove for 3–5 minutes. The pot is already too hot to safely touch, while the water inside the pot has barely increased its temperature. Compared to many other liquids, water requires an extraordinary amount of heat energy to be absorbed before changing its temperature. This makes water an excellent heat sink within the human body, as it is able to absorb the heat generated by chemical reactions without greatly increasing our body temperature. Moreover, when environmental temperature soars, the water stored in the body helps keep the body cool. This cooling effect happens as warm blood from the body’s core flows through blood vessels just under the skin and is released out to the environment as radiant heat. At the same time, sweat glands secrete warm water in sweat. For evaporation of this water to occur, the hydrogen bonds between the individual water molecules must be broken, requiring a relatively high amount of energy that in part includes heat. This removal of heat by evaporation results in a cooling of the blood in the body’s periphery, near the surface of the skin, which then circulates back to the body core and cools the body. As the hottest sweat molecules evaporate from the skin’s surface, this leaves behind only the cooler sweat molecules, thus decreasing the overall temperature of the skin’s surface.
Water as a Component of Liquid Mixtures
A mixture is a combination of two or more substances, each of which maintains its own chemical identity. In other words, the constituent substances are not chemically bonded into a new, larger chemical compound. This concept is easy to imagine if you think of powdery substances such as flour and sugar. When stirred together in a bowl, they obviously do not bond to form a new compound. The air you breathe is a gaseous mixture, containing the element argon, molecules of nitrogen and oxygen, and one compound—carbon dioxide.
For cells in the body to survive, they must be kept moist in a water-based liquid called a solution. In chemistry, a liquid solution consists of a solvent that dissolves a substance called a solute. An important characteristic of solutions is that they are homogeneous; that is, the solute molecules are distributed evenly throughout the solution.
The Role of Water in Chemical Reactions
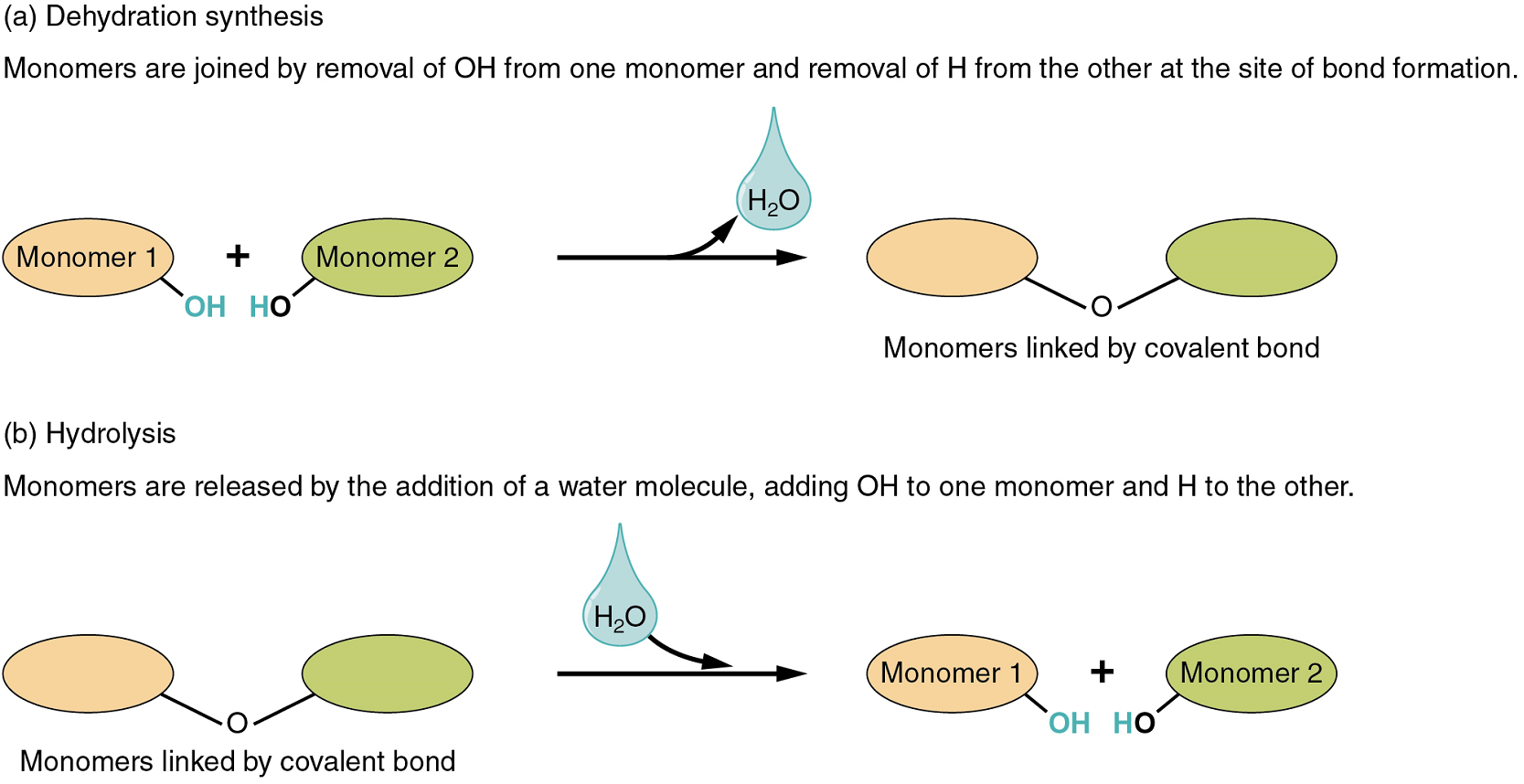
Two types of chemical reactions involve the creation or the consumption of water: dehydration synthesis and hydrolysis.
- During dehydration synthesis, one monomer donates an atom of hydrogen, and another monomer donates a hydroxyl group (OH). These monomers then form a covalent bond between themselves. In the formation of their covalent bond, a molecule of water is released as a by-product (Figure 1). This process is also sometimes referred to as a condensation reaction.
- During hydrolysis, a molecule of water disrupts a compound, breaking its covalent bond. The water is itself split into H+ and OH−. One portion of the severed compound then bonds with the hydrogen atom, and the other portion bonds with the hydroxyl group.
Both of these reactions are reversible and play an important role in the chemistry of organic compounds (which will be discussed shortly).
Test Your Knowledge
For each of the four biologically important properties of water:
- Identify the property.
- Describe its importance in the human body.

Watch this Crash Course video to learn more about the importance of water and its chemical properties. Direct link: https://youtu.be/HVT3Y3_gHGg

Watch this Amoeba Sisters video to learn more about the properties of water! Direct link: https://youtu.be/3jwAGWky98c
Part 2. Fluid Compartments in the Human Body
Body Water Content
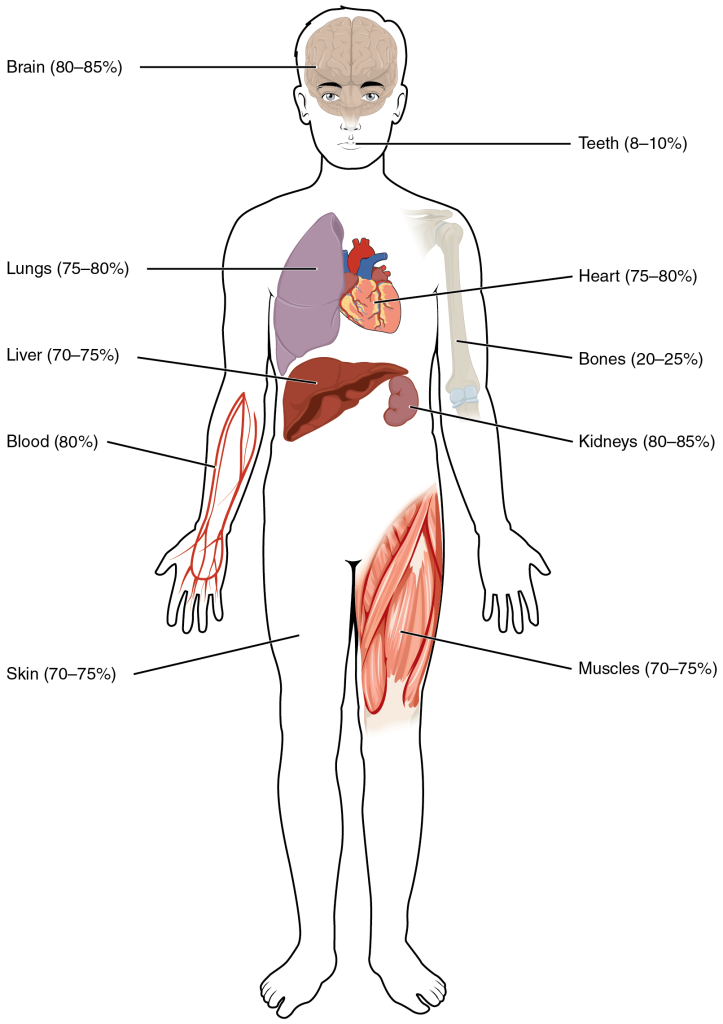
The human body is mostly water, ranging from about 75% of body mass in infants to as low as 45% in elderly individuals. In adults, the average percent of body mass in women is 50%, whereas in men, the average is 60%. The percent of body water changes with development, because the proportions of the body given over to each organ and to muscles, fat, bone, and other tissues change from infancy to adulthood (Figure 2).
Fluid Compartments
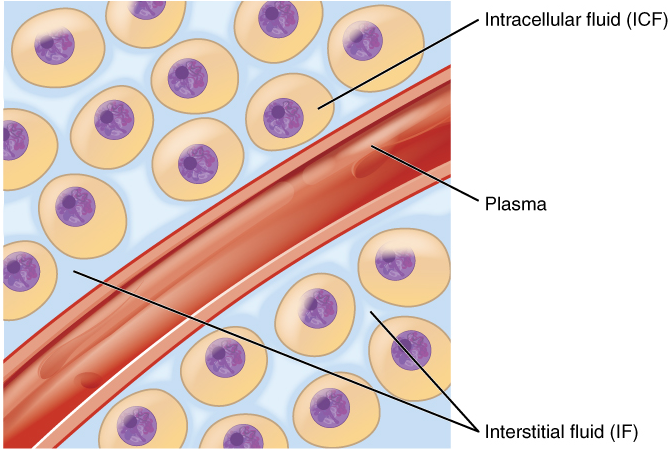
Body fluids can be discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the body. This fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells not located in the blood (Figure 3).
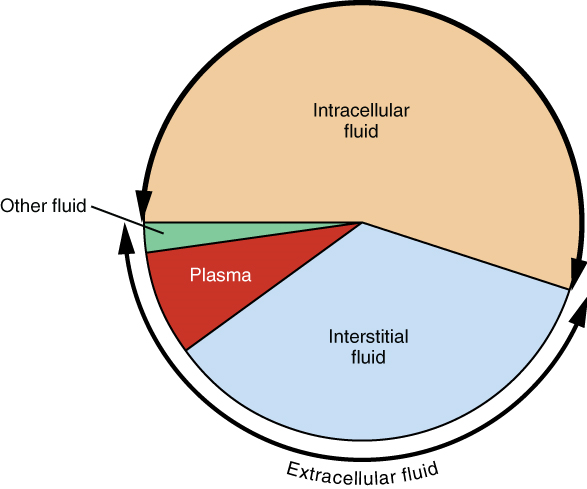
1. Intracellular Fluid: The intracellular fluid lies within cells and is the principal component of the cytosol. The intracellular fluid makes up approximately 60% of the total water in the human body, and in an average-size adult male, the intracellular fluid accounts for about 25 liters (seven gallons) of fluid (Figure 4). This fluid volume tends to be very stable, because the amount of water in living cells is closely regulated. If the amount of water inside a cell falls to a value that is too low, the cytosol becomes too concentrated with solutes to carry on normal cellular activities. If too much water enters a cell, the cell may burst.
2. Extracellular Fluid: The extracellular fluid accounts for the other approximately 40% of the body’s water content. About half of the extracellular fluid is found in plasma. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Gases, nutrients, and waste materials travel between capillaries and cells through the interstitial fluid. Cells are separated from the interstitial fluid by a selectively permeable cell membrane that helps regulate the passage of materials between the interstitial fluid and the interior of the cell.
The body has other water-based extracellular fluids. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, synovial fluid in joints, pleural fluid in the pleural cavities, pericardial fluid in the cardiac sac, peritoneal fluid in the peritoneal cavity, and aqueous humor of the eye. Because these fluids are outside cells, these fluids are also considered components of the extracellular fluid compartment.
Test Your Knowledge
- Given your (approximate) body weight, calculate the amount of water you contain in liters. You may use a calculator if necessary but must clearly show all your work
-
For each of the major fluid compartments of the human body:
- Name the compartment.
- Define the compartment by specifying its location in the human body.
- Specify the percentage of body fluid volume made up by that compartment.
Practice
For the following activities, drag and match the answers to the correct empty boxes on the images.
Image Descriptions
Figure 4.2 image description: The figure shows these different organs and tissues and percentage of their total mass that is accounted for by water. The brain is 80–85%, lungs are 75–80%, the liver is 70–75%, blood is 80%, skin is 70–75%, teeth are 8–10%, the heart is 75–80%, bones are 20–25%, kidneys are 80–85%, and muscles are 70–75%. [Return to image.]
Figure 4.4 image description: The figure illustrates the proportions of total body fluid in each of the body’s fluid compartments. Over half of the total water in the body is intracellular fluid. The second largest volume is the interstitial fluid, which surrounds non-blood cells. [Return to image.]
Thick, lubricating fluid that fills the interior of a synovial joint.
Substance that acts as a lubricant for the visceral and parietal layers of the pleura during the movement of breathing.
Scatter or break up.
Dipole-dipole bond in which a hydrogen atom covalently bonded to an electronegative atom is weakly attracted to a second electronegative atom.
A substance composed of two or more different elements joined by chemical bonds.
In chemistry, a homogeneous liquid mixture in which a solute is dissolved into molecules within a solvent.
Component of a solution, the substance that dissolves the solute.
Component of a solution, the substance dissolved in a solvent.
Condition in which solute molecules are distributed equally in a solution.
Chemical reaction in which reactants combine to form a new compound, with one reactant giving up an atom of hydrogen and another reactant giving up a hydroxyl group (OH).
A functional group, OH, present in many organic compounds including alcohols.
Chemical reaction in which a molecule water is split into H and OPH, thereby breaking a bond and severing a compound.
Fluid inside cells.
Fluid outside cells (plasma or interstitial fluid).
An extracellular fluid, the fluid component of blood.
Clear, semi-fluid medium of the cytoplasm, made up mostly of water.
(Also, coagulation factors) group of 12 identified substances active in coagulation.
(Also, immunoglobulin) antigen-specific protein secreted by plasma cells.
A solution containing ions; sometimes referring to ions themselves.
Smallest of the blood vessels where physical exchange occurs between the blood and tissue cells surrounded by interstitial fluid.
Extracellular fluid in the small spaces between cells not contained within blood vessels.
Feature of any barrier that allows certain substances to cross but excludes others.
Circulatory medium within the CNS that is produced by ependymal cells in the choroid plexus filtering the blood.
Fluid contained within the lymphatic system, consisting of interstitial fluid, leukocytes (white blood cells), proteins (including antibodies), and fats.
The space between the visceral and parietal pleurae.
Fluid found in the pericardium.
Cavity surrounding the heart filled with a lubricating serous fluid that reduces friction as the heart contracts (also, pericardial cavity or cardiac sac).

