Jonathan Akin
Unit Outline
Part 1: Nervous Stimulus to Skeletal Muscular Contraction
Learning Objectives
At the end of this unit, you should be able to:
I. Describe the anatomy of a neuromuscular junction.
II. Explain the process of muscle contraction.
III. Describe the physiology of muscle relaxation.
IV. Describe the concept of muscle tone as it pertains to skeletal muscle.
V. Define the following terms: paralysis, muscular dystrophy, muscular atrophy, muscular hypertrophy.
VI. Describe the microscopic anatomy (histology) of cardiac muscle.
VII. Describe the mechanism of contraction in cardiac muscle. Describe in detail how a cardiac muscle contracts by describing the events that occur within the cardiac muscle starting from the depolarization of the plasma membrane of a cardiac muscle cell and ending with cross-bridge formation.
VIII. Describe the functional significance of self-excitatory cardiac muscle cells.
IX. Describe the microscopic anatomy of smooth muscle.
X. Explain the mechanism of contraction and relaxation in smooth muscle.
XI. Describe the chemical factors that regulate contraction of smooth muscle.
XII. Define the process and anatomical basis of peristalsis.
Imagine the process of performing an arm curl resistance exercise when you pick up a dumbbell. Your biceps brachii will contract to resist the weight of the dumbbell and, if you have enough strength, flex the arm. What caused the biceps muscle to begin contracting? If you do repetitions of this exercise, can you do this indefinitely? How fast will fatigue set in and why? If you change resistance levels by switching to heavier or lighter weights, how does that affect the contraction? Once you are finished exercising, how do muscles relax?
Contraction or shortening is the chief function of all muscle types, not just skeletal muscle. The beginning of this chapter explains how voluntary control of skeletal muscle works and how striated muscle contracts based on the sliding filament model. Muscle strength can change through exercise (i.e., training), and different muscles show varying degrees of resistance to fatigue. One cause of fatigue is ATP availability, and how muscles tap different sources of energy will be examined. The chapter finishes by looking at the actions of cardiac and smooth muscle.
Part 1: Nervous Stimulus to Skeletal Muscular Contraction
Two requirements must be met in order for skeletal muscular contraction to occur. First, there must be in place a neuromuscular junction.
The Neuromuscular Junction
The neuromuscular junction is the site where a motor neuron’s terminal forms a synapse with a muscle fiber (the equivalent of a single muscle cell, as recalled from Chapter 13, Muscle Anatomy and Movement). The neuromuscular junction is where the muscle fiber first responds to electrochemical signaling by the motor neuron. Every skeletal muscle fiber must be activated, or stimulated, by a motor neuron at the neuromuscular junction so that a change in membrane potential occurs.

Excitation signals from the neuron are the only way to functionally activate the muscle fiber to contract; they generate an electrical impulse, called an action potential, in the sarcolemma (plasma membrane) of the muscle fiber.
The action potential that is generated results in contraction, which is why the second requirement for skeletal muscular contraction is referred to as excitation-contraction coupling.
Excitation-Contraction Coupling
All living cells have membrane potentials, or electrical gradients, across their membranes. The inside of the plasma membrane of most cells is usually around -70 mV, relative to the outside. This is referred to as a cell’s resting membrane potential. Neurons and muscle cells can use their membrane potentials to generate electrical signals. They do this by controlling the movement of charged particles, called ions, across their membranes to create electrical currents. This is achieved by opening and closing specialized proteins in the membrane called ion channels. Although the currents generated by ions moving through these channel proteins are very small, they form the basis of both neural signaling and muscle contraction.
Both neurons and skeletal muscle cells are electrically excitable, meaning that they are able to change their membrane potential in a regulated fashion. Large changes in membrane potential often lead to the production of action potentials. An action potential is a special type of electrical signal that can propagate along a cell membrane as a wave. While action potentials were originally believed to travel, in reality, they are actually regenerated repeatedly along small stretches of membrane. This process is called propagation. It is this propagation that allows a signal to be transmitted quickly and faithfully over long distances.
Although the term excitation-contraction coupling confuses or scares some students, it comes down to this: for a skeletal muscle fiber to contract, its membrane must first be “excited”—in other words, it must be stimulated to fire an action potential. The muscle fiber action potential, which sweeps along the sarcolemma as a wave, is “coupled” to the actual contraction through the release of calcium ions from the sarcoplasmic reticulum(SR).
In skeletal muscle, this sequence begins with signals from the somatic motor division of the nervous system. In other words, the “excitation” step in skeletal muscles is always triggered by signaling from the nervous system (Figure 14.1).
Signaling begins when a neuronal action potential propagates along the axon of a motor neuron and then along the individual branches of the axon, terminating at individual neuromuscular junctions. Membrane potential changes cause calcium channels in the membrane of the neuron to open, allowing calcium ions to diffuse into the neuron’s cytosol. This influx of Ca2+ causes vesicles in the neuron to fuse with the plasma membrane, releasing their contents—the neurotransmitter Acetylcholine (ACh)—into the space between the neuron and the muscle fiber, called the synaptic cleft. It is the exocytotic release of acetylcholine from these vesicles that is ultimately calcium-ion dependent.
Thus, the associated axon terminal at each neuromuscular junction releases Acetylcholine (ACh). The acetylcholine molecules diffuse across a minute space called the synaptic cleft and bind to acetylcholine receptors located within the motor end-plate of the sarcolemma on the other side of the synapse. The motor end-plate is simply a region of skeletal muscle membrane with a dense population of receptors for acetylcholine. Once acetylcholine binds to these receptors, a channel in the acetylcholine receptor (called a ligand-gated ion channel) opens, and positively charged ions are able to enter the muscle fiber, causing it to depolarize, meaning that the membrane potential of the muscle fiber becomes less negative (closer to zero, and this continues so that there is a temporary reversal of charge with the inside of the membrane briefly positive relative to the outside).
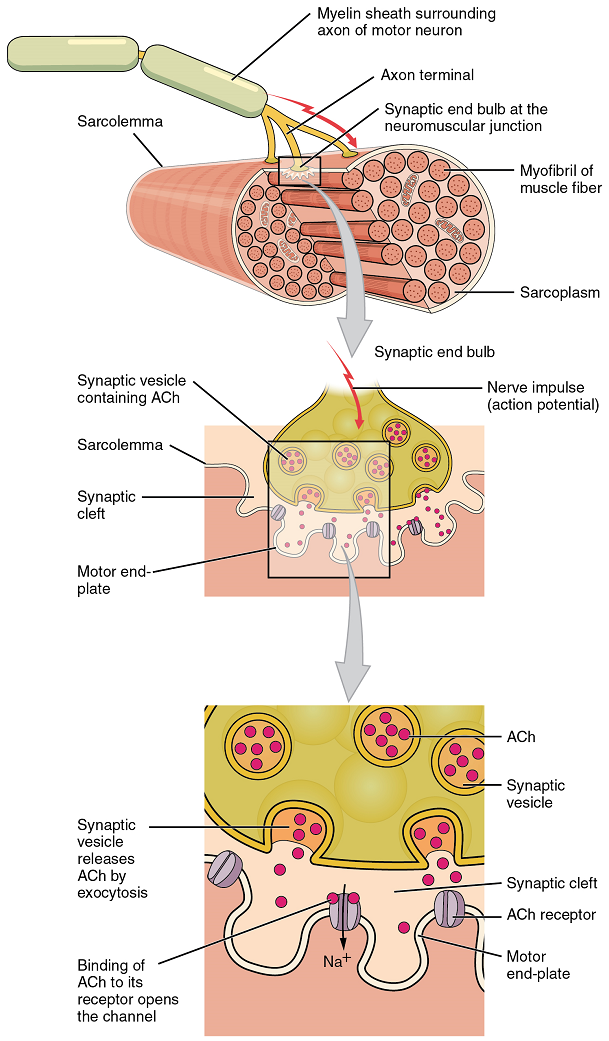
When the membrane is depolarized to a specific value, another set of ion channels called voltage-gated sodium channels are triggered to open. Sodium ions enter the muscle fiber, and an action potential rapidly spreads (or “fires”) along the entire membrane to initiate excitation-contraction coupling.
Things happen very quickly in the world of excitable membranes (just think about how quickly you can snap your fingers as soon as you decide to do it). Immediately following depolarization of the membrane, it repolarizes, re-establishing the negative membrane potential. Meanwhile, the acetylcholine in the synaptic cleft is degraded by the enzyme acetylcholinesterase (AChE) so that the acetylcholine can no longer bind to an acetylcholine receptor, thereby avoiding unwanted muscle excitation and contraction.
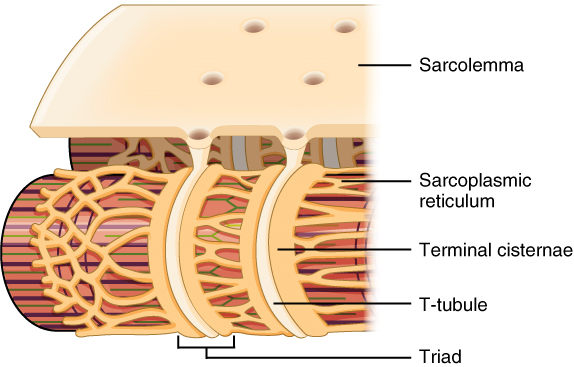
Propagation of an action potential along the sarcolemma is still part of the excitation portion of excitation-contraction coupling; it is this excitation that triggers the release of calcium ions from its storage in the cell’s sarcoplasmic reticulum. For the action potential to reach the membrane of the SR, there are periodic invaginations that run deep within the sarcolemma, called transverse tubules (T-tubules). A T-tubule along with SR membranes on either side of it is referred to as a triad (Figure 14.2). Triads surround and enclose the cylindrical structure known as a myofibril, which contains actin and myosin, commonly referred to as the contractile machinery of a muscle fiber.
T-tubules thus transmit the action potential into the interior of the cell. The action potential triggers the opening of calcium channels in the membrane of adjacent SRs, causing calcium ions to diffuse out of the SR and into the sarcoplasm. It is the increase in concentration of Ca2+ in the sarcoplasm that initiates contraction of the muscle fiber by its contractile units, or sarcomeres.
Test Your Knowledge
I. Describe the anatomy of a neuromuscular junction.
- Draw a fully labeled diagram showing a neuromuscular junction. Add annotations explaining the events that occur when a nerve impulse (or action potential) arrives at the synaptic end bulb, until the time the sarcolemma of the muscle cell is depolarized.
Part 2: Skeletal Muscle Tissue
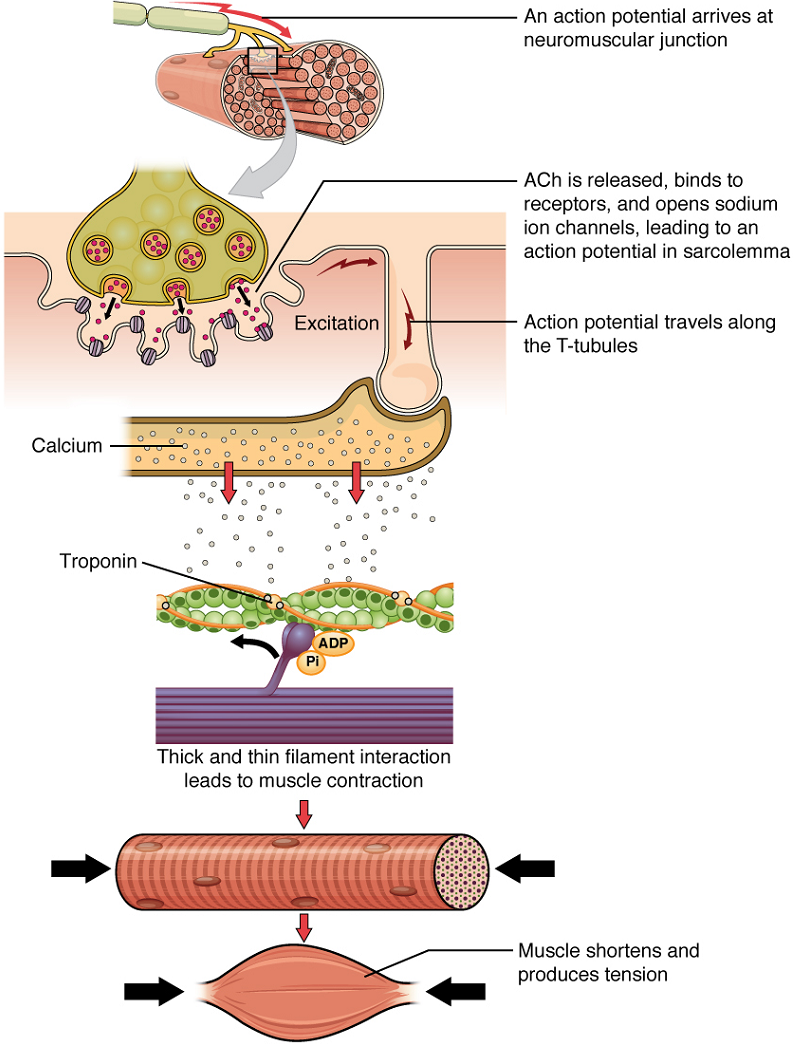
Once again, the sequence of events that result in the contraction of an individual muscle fiber begins with an electrical signal—an action potential—propagating down a motor neuron, innervating the muscle fiber. Calcium’s role in initiating the release of acetylcholine from the synaptic end bulb has already been described.
When acetylcholine reaches the muscle fiber’s sarcolemma, it binds to closed acetylcholine-gated ion channels that now open as a result. In the area where these ion channels open, the sarcolemma of the muscle fiber will depolarize as positively charged sodium ions (Na+) enter, triggering an action potential that spreads to the rest of the sarcolemma. The whole sarcolemma will depolarize, including the T-tubules.
Embedded in the walls of the T-tubules of skeletal muscle fibers are voltage-sensitive proteins that are connected to calcium channels in the membrane of the adjacent sarcoplasmic reticulum (SR). When the action potential travels along each T-tubule, the voltage-sensitive proteins there change shape, pulling open the gate on the calcium channels on the SR. This allows Ca2+ ions to be released from their storage in the SR (where their concentration is higher), out to the cytosol (sarcoplasm) of the muscle fiber. The Ca2+ ions then initiate contraction, which is sustained by ATP (Figure 14.3).
Troponin and tropomyosin are the two major proteins that regulate skeletal muscle contraction. Tropomyosin is a long, rod-like molecule that shields (blocks) the myosin-binding sites on actin. Troponin, which has a binding site for Ca2+, is a Ca2+-sensitive globular protein whose role is to keep the longer tropomyosin in its place.
Upon the binding of Ca2+ to troponin, troponin changes its conformation (overall three-dimensional shape) and loses its hold on tropomyosin, thereby exposing the myosin-binding sites on actin.
Therefore, as long as calcium ions remain in the sarcoplasm to bind to troponin, which in turn keeps the myosin-binding sites on actin “unshielded,” and as long as ATP is available to drive the cross-bridge cycling and the pulling of actin strands by myosin, the muscle fiber will continue to shorten to an anatomical limit.
Muscle contraction usually stops when signaling from the motor neuron ends, which repolarizes the sarcolemma and T-tubules and closes the calcium channels in the SR. Calcium ions are then pumped (actively transported) back into the SR, which causes the tropomyosin to re-shield (or re-cover) the binding sites on the actin strands. A muscle also can stop contracting when it runs out of ATP and becomes fatigued (Figure 14.4).
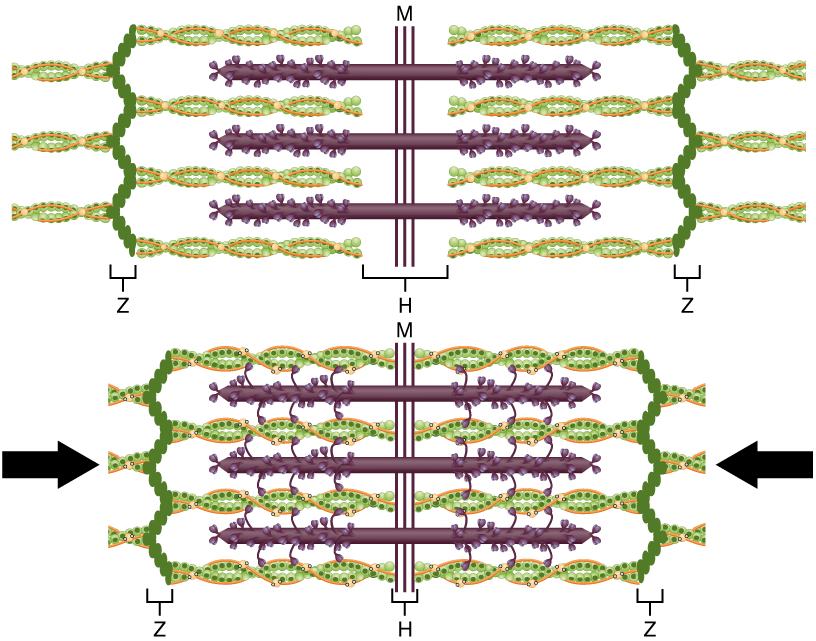
The molecular events of muscle fiber shortening occur within the fiber’s sarcomeres (Figure 14.5). The contraction of a striated muscle fiber occurs as the sarcomeres, linearly arranged within myofibrils, shorten as myosin heads pull on the actin filaments.
The region where thick and thin filaments overlap has a dense appearance, as there is little space between the filaments. This zone, where thin and thick filaments overlap, is very important to muscle contraction, as it is the site where filament movement starts. Thin filaments, anchored at their ends by the Z-discs, do not extend completely into the central region that only contains thick filaments, which are themselves anchored at their bases at a spot called the M line. A myofibril is composed of many sarcomeres running along its length; thus, myofibrils and muscle cells shorten (contract) as the sarcomeres contract.

The Sliding Filament Model
The Sliding Filament Model of Contraction: When signaled by a motor neuron, a skeletal muscle fiber contracts as the thin filaments are pulled and then slide past the thick filaments within the fiber’s sarcomeres. This process is known as the sliding filament model of muscle contraction (Figure 14.5). The sliding can only occur when myosin-binding sites on the actin filaments are exposed by a series of steps that begins with Ca2+ entry into the sarcoplasm.
Recall that to initiate muscle contraction, tropomyosin has to expose the myosin-binding site on an actin filament to allow a cross-bridge formation between the actin and myosin microfilaments. The first step in the process of contraction is for Ca2+ to bind to troponin so that tropomyosin can slide away from the binding sites on the actin strands. This allows the myosin heads to bind to these exposed binding sites and form cross-bridges. The thin filaments are then pulled by the myosin heads to slide past the thick filaments toward the center of the sarcomere. But each head can only pull a very short distance before it has reached its limit and must be “re-cocked” before it can pull again, a step that requires ATP.
ATP and Muscle Contraction: For thin filaments to continue to slide past thick filaments during muscle contraction, myosin heads must pull the actin at the binding sites, detach, re-cock, attach to more binding sites, pull, detach, re-cock, etc. This repeated movement is known as the cross-bridge cycle.
Each cross-bridge cycle requires energy, which is provided by ATP.
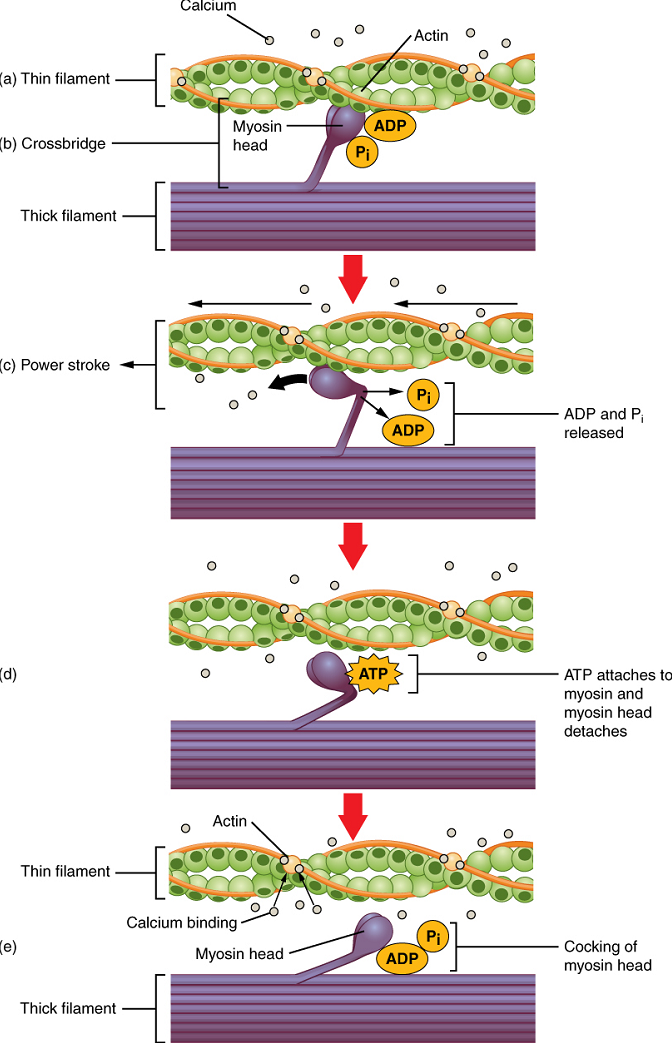
Cross-bridge formation occurs when the myosin head attaches to the actin while adenosine diphosphate (ADP) and inorganic phosphate (Pi) are still bound to myosin (Figure 14.6a,b). Pi is then released, causing myosin to form a stronger attachment to actin, after which the myosin head moves toward the M line, pulling the actin along with it and releasing the ADP. As actin is pulled, the filaments move approximately 10 nm toward the M line. This movement is called the power stroke, as movement of the thin filament occurs at this step (Figure 14.6c). In the absence of ATP, the myosin head will not detach from actin.
One part of the myosin head attaches to the binding site on the actin, but the head has another binding site for ATP. ATP binding causes the myosin head to detach from the actin (Figure 14.6d).
After this occurs, ATP is hydrolyzed into ADP and Pi by the intrinsic ATPase activity of myosin. The energy released during ATP hydrolysis changes the angle of the myosin head into a cocked position (Figure 14.6e). When the myosin head is cocked, it is said to be in a high-energy configuration and is capable of further movement as long as ATP is available.
Note that each thick filament of roughly 300 myosin molecules has multiple myosin heads, and many cross-bridges form and break continuously during muscle contraction. Multiply this by all of the sarcomeres in one myofibril, all the myofibrils in one muscle fiber, and all of the muscle fibers in one skeletal muscle, and you can understand why so much energy (ATP) is needed to keep skeletal muscles working. In fact, it is the loss of ATP that results in the rigor mortis observed soon after someone dies. With no further ATP production possible, there is no ATP available for myosin heads to detach from the actin-binding sites, so the cross-bridges stay in place, causing rigidity in the skeletal muscles.
Relaxation of Skeletal Muscle
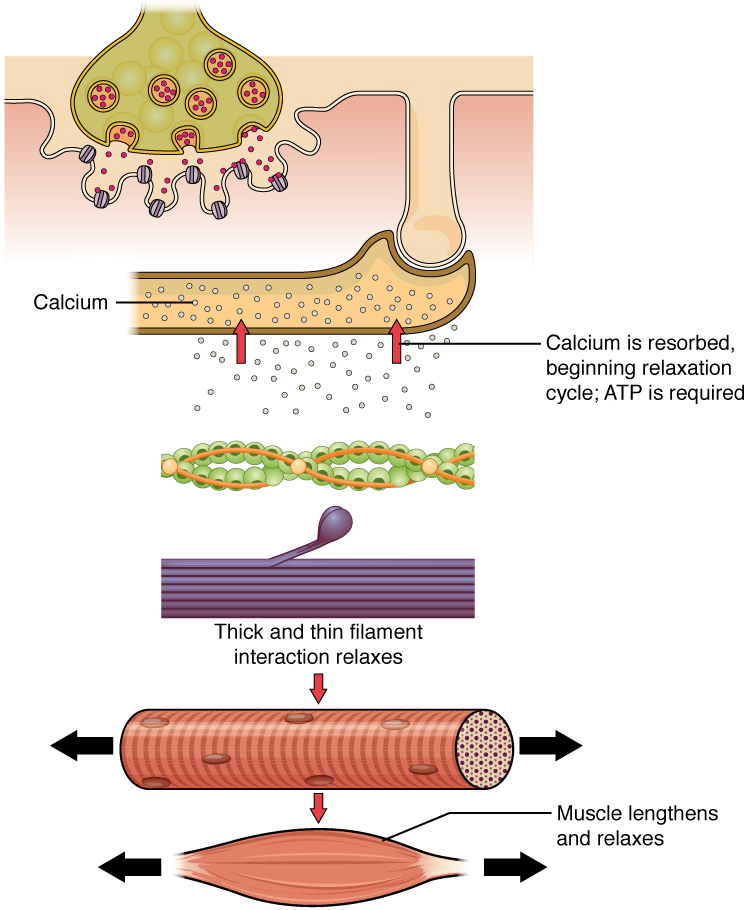
Relaxation of a Skeletal Muscle: Relaxing skeletal muscle fibers, and ultimately, the skeletal muscle, begins with the motor neuron, which stops releasing its chemical signal, acetylcholine, into the synapse at the neuromuscular junction. The muscle fiber will repolarize, which closes the channels in the SR where Ca2+ was being released. ATP-driven pumps will move Ca2+ out of the sarcoplasm back into the SR. Ca2+ no longer binds to troponin, resulting in the “re-shielding” of the myosin-binding sites on the thin filaments by tropomyosin, which is now once again held in its place by troponin. Without the ability to form cross-bridges between the thin and thick filaments, the muscle fiber loses its tension and relaxes.
Muscle Tone: Skeletal muscles are rarely completely relaxed, or flaccid. Even if a muscle is not producing movement, it is contracted a small amount to maintain its contractile proteins and produce muscle tone. This continuous partial contraction of a muscle that causes the muscle to resist passive stretch while at rest is referred to as muscle tone. The tension produced by muscle tone allows muscles to continually stabilize joints and maintain posture.
Muscle tone is accomplished by a complex interaction between the nervous system and skeletal muscles that results in the activation of a few motor units at a time, most likely in a cyclical manner. In this manner, muscles never fatigue completely, as some motor units can recover while others are active.
The absence of the low-level contractions that lead to muscle tone is referred to as hypotonia or atrophy and can result from damage to parts of the central nervous system, such as the cerebellum, or from loss of innervations to a skeletal muscle, as in poliomyelitis. Hypotonic muscles have a flaccid appearance and display functional impairments, such as weak reflexes or flaccid paralysis, where a person loses the ability to move affected muscles of the body. Conversely, excessive muscle tone is referred to as hypertonia, accompanied by hyperreflexia (excessive reflex responses), often the result of damage to upper motor neurons (found in the cerebral cortex and brain stem) of the central nervous system. Hypertonia can present with muscle rigidity (as seen in Parkinson’s disease) or spasticity, a phasic change in muscle tone, where a limb will “snap” back from passive stretching (as seen in some strokes). Severe cases of this condition can lead to a specific type of paralysis, called spastic paralysis.
Sources of ATP Used by Muscle
Sources of ATP: ATP supplies the energy for muscle contraction to take place. In addition to its direct role in the cross-bridge cycle, ATP also provides the energy for the active transport utilizing Ca2+ pumps housed in the SR membranes. Muscle contraction does not occur without sufficient amounts of ATP. The amount of ATP stored in muscle is very low, only sufficient to power a few seconds worth of contractions.
Therefore, as it is broken down, ATP must be regenerated and replaced quickly to allow for sustained contraction: ATP can be regenerated through three mechanisms: creatine phosphate metabolism, anaerobic pathway (glycolysis and lactic acid formation), and aerobic cellular respiration.
(a) Creatine phosphate is a molecule that can store energy in its phosphate bonds. In a resting muscle, excess ATP transfers its energy to creatine, producing ADP and creatine phosphate. This acts as an energy reserve that can be used to quickly create more ATP. When the muscle starts to contract and needs energy, creatine phosphate transfers its phosphate back to ADP to form ATP and creatine. This reaction is catalyzed by the enzyme creatine kinase and occurs very quickly; thus, creatine phosphate-derived ATP powers the first few seconds of muscle contraction. However, creatine phosphate can only provide approximately 15 seconds worth of energy, at which point another energy source has to be used (Figure 14.7).
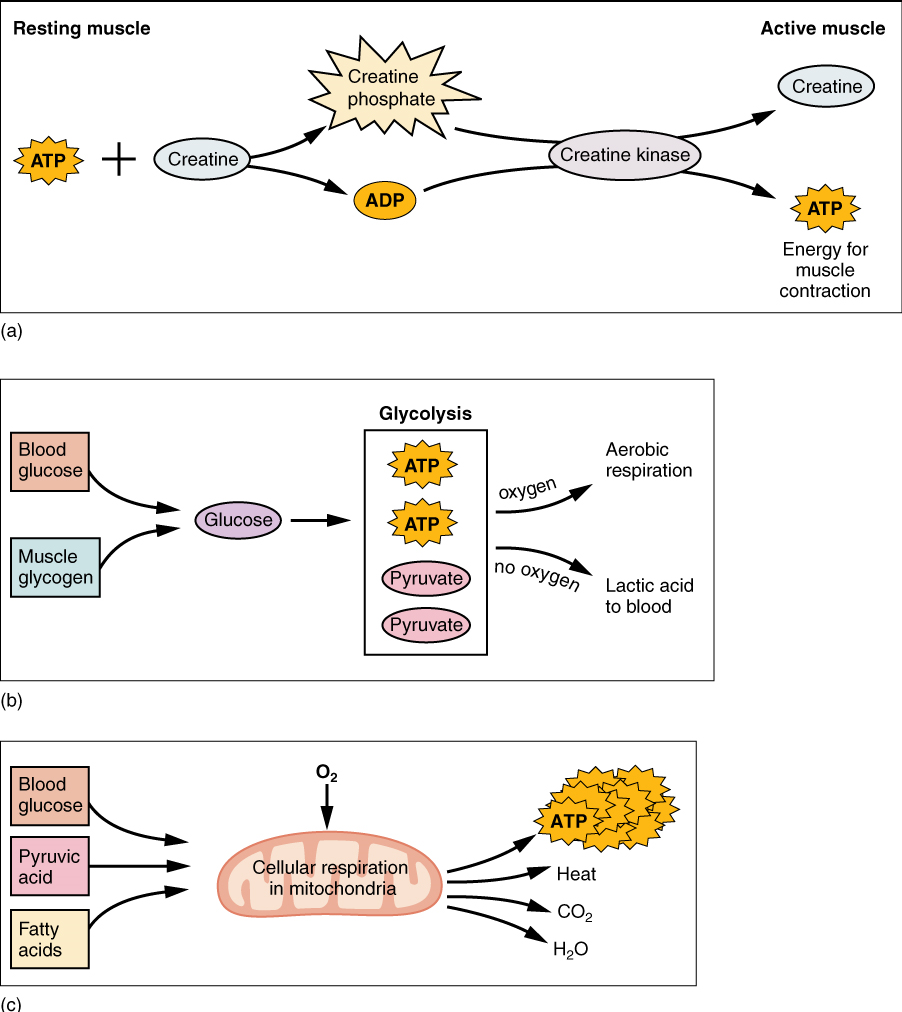
(b) The Anaerobic Pathway: As the ATP produced by creatine phosphate is depleted, muscles turn to glycolysis as an ATP source. Glycolysis is an anaerobic (non-oxygen-dependent) process that breaks down glucose (sugar) to produce ATP. Because glycolysis cannot generate ATP as quickly as creatine phosphate, the switch to glycolysis results in a slower rate of ATP availability to the muscle. The sugar used in glycolysis can be provided by blood glucose or by metabolizing glycogen that is stored in the muscle. The initial breakdown of one glucose molecule produces two ATP and two molecules of pyruvic acid, which can be used either in aerobic respiration if sufficient oxygen is available or when oxygen levels are low, converted to lactic acid (Figure 14.7b).
The lactic acid so produced may contribute to muscle fatigue. This conversion allows the recycling of the coenzyme NAD+ from NADH, which is needed for glycolysis to continue. This occurs during strenuous exercise when high amounts of energy are needed but oxygen cannot be sufficiently delivered to muscle. Glycolysis itself cannot be sustained for very long (approximately one minute of muscle activity), but it is useful in facilitating short bursts of high-intensity output. This is because glycolysis does not utilize glucose very efficiently, producing a net gain of two ATPs per molecule of glucose, and the end product of lactic acid in the absence of oxygen.
(c) Aerobic cellular respiration is the breakdown of glucose or other nutrients in the presence of oxygen to produce carbon dioxide, water, and ATP. Approximately 95% of the ATP required for resting or moderately active muscles is provided by aerobic respiration, which takes place in mitochondria. The inputs for aerobic respiration include glucose circulating in the bloodstream, pyruvic acid, and fatty acids. Aerobic respiration is much more efficient than anaerobic glycolysis, producing approximately 32 to 34 ATPs per molecule of glucose versus two (net) from glycolysis. However, aerobic respiration cannot be sustained without a steady supply of O2 to the skeletal muscle (Figure 14.7c). To compensate, muscles store a small amount of excess oxygen in a protein called myoglobin, allowing for more efficient muscle contractions and less fatigue. Aerobic training also increases the efficiency of the circulatory system so that O2 can be supplied to the muscles for longer periods of time.
Exercise and Muscle Performance
Exercise and Muscle Performance: Physical training alters the appearance of skeletal muscles and can produce changes in muscle performance. Conversely, a lack of use can result in decreased performance and muscle appearance. Although muscle cells can change in size, new cells are not formed at adulthood when muscles grow. Instead, structural proteins are added to muscle fibers in a process called hypertrophy, so cell diameter increases. The reverse, when structural proteins are lost and muscle mass decreases, is called atrophy. Age-related muscle atrophy is called sarcopenia. Cellular components of muscles can also undergo changes in response to changes in muscle use.
Muscle Atrophy: Although atrophy due to disuse can often be reversed with exercise, muscle atrophy can also be the result of any of a number of genetic diseases, called muscular dystrophy, that result in increasing weakness of muscles and loss of muscle tissue over time. Although there are medications that can slow muscle degeneration and reduce damage to dying muscle cells, the atrophy due to muscular dystrophy is irreversible. Muscle atrophy with age, referred to as sarcopenia, is also irreversible. This is a primary reason why even highly trained athletes succumb to declining performance with age. This decline is noticeable in athletes whose sports require strength and powerful movements, such as sprinting, whereas the effects of age are less noticeable in endurance athletes such as marathon runners or long-distance cyclists. As muscles age, muscle fibers die, and they are replaced by connective tissue and adipose tissue (Figure 14.8). Because those tissues cannot contract and generate force as muscle can, muscles lose the ability to produce powerful contractions. The decline in muscle mass causes a loss of strength, including the strength required for posture and mobility. This may be caused by a reduction in FG (Fast Glycolytic) fibers that hydrolyze ATP quickly to produce short, powerful contractions. Muscles in older people sometimes possess greater numbers of SO (Slow Oxidative) fibers, which are responsible for longer contractions and do not produce powerful movements. There may also be a reduction in the size of motor units, resulting in fewer fibers being stimulated and less muscle tension being produced.
Sarcopenia can be delayed to some extent by exercise, as training adds structural proteins and causes cellular changes that can offset the effects of atrophy. Increased exercise can produce greater numbers of cellular mitochondria, increase capillary density, and increase the mass and strength of connective tissue.
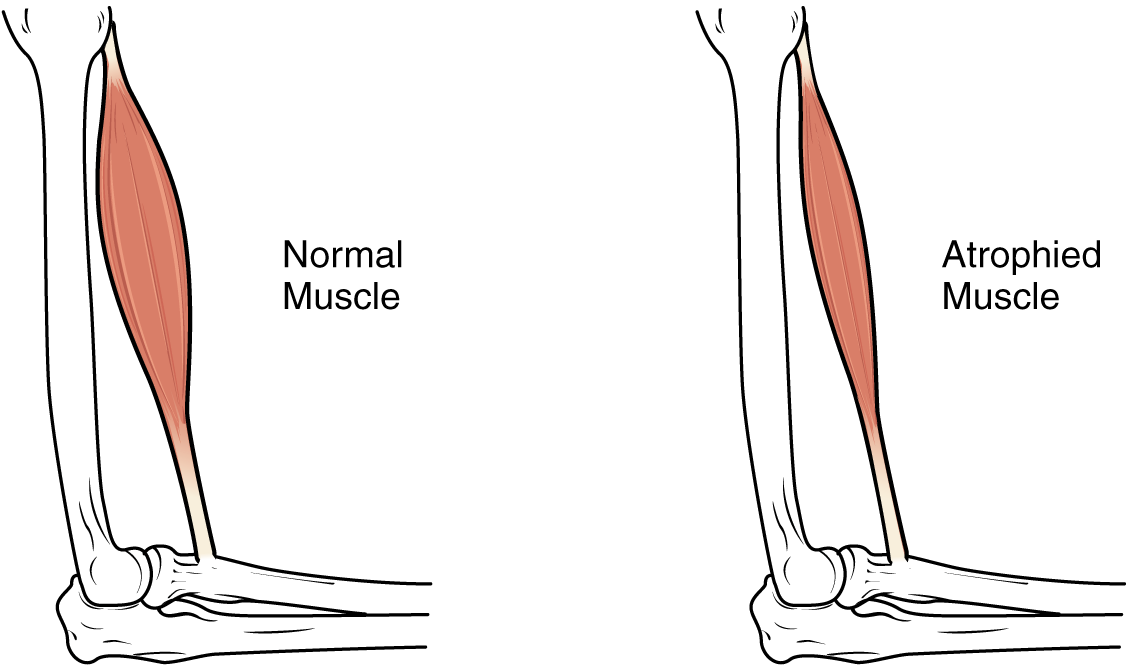
The effects of age-related atrophy are especially pronounced in people who are sedentary, as the loss of muscle cells is displayed as functional impairments such as trouble with locomotion, balance, and posture. This can lead to a decrease in quality of life and medical problems, such as joint problems, because the muscles that stabilize bones and joints are weakened. Problems with locomotion and balance can also cause various injuries due to falls.
Test Your Knowledge
II. Explain the process of muscle contraction.
- Describe the physiology of muscle contraction, including the roles of the following components: calcium ions (Ca2+), troponin, tropomyosin, myosin, actin, and ATP.
- Describe the generation of a muscle action potential, including the roles of acetylcholine (ACh) and sodium (Na+) ions.
- Draw and fully label a diagram showing one fully contracted sarcomere. Your diagram must include the following labeled structures:
- Z line
- A band
- M line
- I band
- H zone
- Sarcomere width
- Complete your diagram above by annotating it. For each of the labeled structures above, add notes that state and explain how that particular structure is different (or not) in a fully contracted vs. a fully relaxed sarcomere:
- Explain the roles of calcium ions in muscle contraction, including their involvement in:
- The release of acetylcholine (ACh) from a motor neuron
- The binding of myosin to actin
- Draw a fully annotated diagram describing how an action potential in a muscle fiber causes the release of calcium ions from the sarcoplasmic reticulum to the sarcoplasm.
- Describe the role of each of the following in the process of muscle contraction: calcium ions, troponin, tropomyosin, myosin, actin, and ATP.
III. Describe the physiology of muscle relaxation.
- Describe the events that must occur for a contracted muscle to relax. Include in your description the events that must occur at:
- The neuromuscular junction
- The sarcomere
- The sarcoplasmic reticulum membrane
- Describe the functions of ATP in a muscle cell, including its roles in muscle contraction, in muscle relaxation, and in maintaining the muscle cell in its resting (relaxed) state.
- List and describe in general terms the three pathways by which a muscle fiber can produce ATP.
- Compare and contrast the three pathways by which a muscle fiber can produce ATP, in terms of:
- The source of the phosphate group attached to ADP to produce ATP
- The name of the substrate (starting molecule) required
- The number of ATP molecules generated per molecule of substrate
- The speed at which ATP can be generated
- Whether myoglobin is useful for each pathway
IV. Describe the concept of muscle tone as it pertains to skeletal muscle.
- Define the term “muscle tone.”
- What is the physiological purpose of this phenomenon?
V. Define the following terms: paralysis, muscular dystrophy, muscular atrophy, and muscular hypertrophy.
- Define the term “paralysis.”
- Briefly describe the major differences between flaccid paralysis and spastic paralysis.
- Describe the common characteristic of the group of muscle diseases known as “muscular dystrophies.”
- Define the following terms:
- Muscular atrophy
- Muscular hypertrophy
Part 3: Cardiac Muscle Tissue
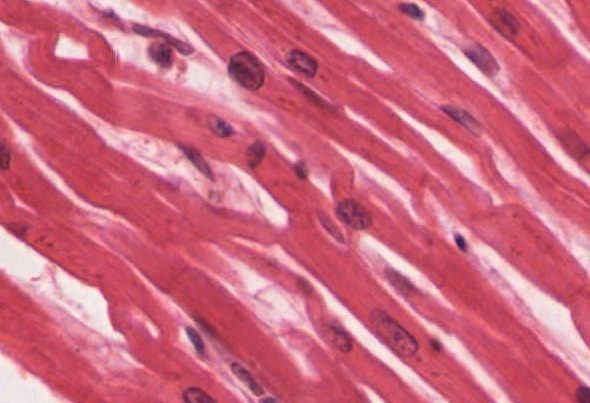
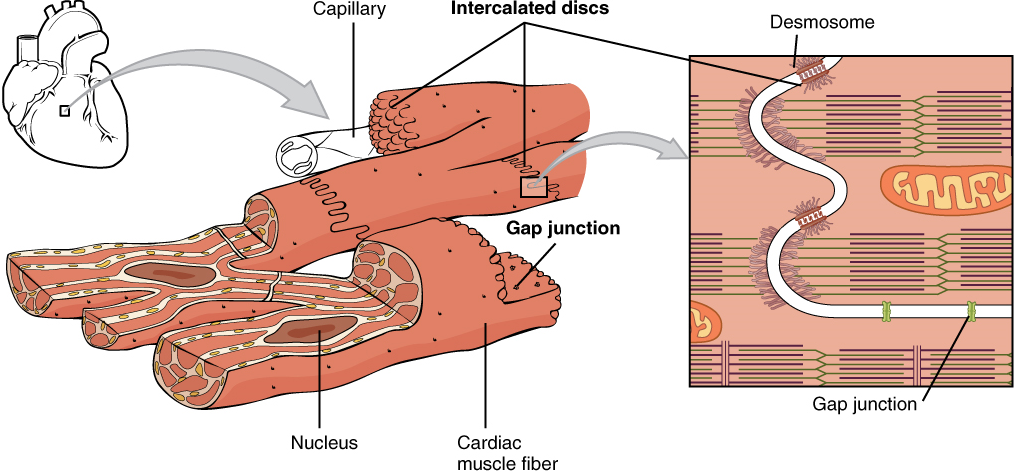
Cardiac muscle tissue is only found in the heart. Highly coordinated contractions of cardiac muscle pump blood into the vessels of the circulatory system. Similar to skeletal muscle, cardiac muscle is striated and organized into sarcomeres, possessing the same banding organization as skeletal muscle (Figure 14.9). However, cardiac muscle fibers are shorter than skeletal muscle fibers and usually contain only one nucleus, which is located in the central region of the cell. Cardiac muscle fibers, similarly to skeletal muscle fibers, also possess many mitochondria and myoglobin, as ATP is produced primarily through aerobic metabolism. Cardiac muscle fiber cells are also extensively branched and are connected to one another at their ends by intercalated discs. An intercalated disc allows the cardiac muscle cells to contract in a wave-like pattern so that the heart can work as a pump.
Intercalated discs are part of the sarcolemma and contain two structures important in cardiac muscle contraction: gap junctions and desmosomes. A gap junction forms channels between adjacent cardiac muscle fibers that allow the depolarizing current produced by cations to flow from one cardiac muscle cell to the next. This joining is called electric coupling (as opposed to excitation-contraction coupling), and in cardiac muscle, it allows the quick transmission of action potentials and the coordinated contraction of the entire heart. This network of electrically connected cardiac muscle cells creates a functional unit of contraction called a syncytium. The remainder of the intercalated disc is composed of desmosomes. A desmosome is a cell structure that anchors the ends of cardiac muscle fibers together so the cells do not pull apart during the stress of individual fibers contracting (Figure 14.10).
Contractions of the heart (heartbeats) are controlled by specialized cardiac muscle cells called pacemaker cells that directly control heart rate. Although cardiac muscle cannot be consciously controlled, the pacemaker cells respond to signals from the autonomic nervous system to increase or decrease heart rate. The pacemaker cells can also respond to various hormones with the effect of modulating heart rate and thus also controlling blood pressure.
The functional syncytium (the wave of contraction that allows the heart to work as a unit) begins with the pacemaker cells. This group of cells is self-excitable and able to depolarize to threshold and fire action potentials on their own, a feature called autorhythmicity; they do this at set intervals, which determine heart rate. Because they are connected with gap junctions to surrounding muscle fibers and the specialized fibers of the heart’s conduction system, the pacemaker cells are able to transmit the depolarization to the other cardiac muscle fibers in a manner that allows the heart to contract in a coordinated manner.
In cardiac cells, unlike skeletal muscles, extracellular Ca2+ is required to initiate the release of calcium from the sarcoplasmic reticulum (SR). The SR in cardiac muscle fibers is simpler than that of skeletal muscle fibers, lacking terminal cisterns, and there is no direct physical link between proteins in the T-tubule and proteins in the SR membrane, so the depolarization of the T-tubule membrane cannot directly cause Ca2+ release from the SR. Instead, cardiac muscle cells have voltage-gated calcium channels in the sarcolemma and along the T-tubules that open when the membrane is depolarized, allowing Ca2+ to enter the cardiac muscle fiber from the extracellular fluid. This calcium then causes the opening of calcium-gated calcium channels in the SR membrane that release additional Ca2+ into the sarcoplasm. This mechanism allows cardiac muscle to have a relatively long-lasting depolarization “plateau” in its fibers. This sustained depolarization (and Ca2+ entry) provides for a longer contraction than is produced by an action potential in skeletal muscle. This, in turn, allows for a longer refractory period and makes tetanus impossible in cardiac muscle.
Test Your Knowledge
VI. Describe the microscopic anatomy (histology) of cardiac muscle.
- Describe the structure and location within a cardiac muscle cell of each of the following:
- Intercalated discs
- Sarcomeres
- T-tubules
- Sarcoplasmic reticulum
VII. Describe the mechanism of contraction in cardiac muscle.
- Describe in detail how a cardiac muscle contracts by describing the events that occur within the cardiac muscle starting from the depolarization of the plasma membrane of a cardiac muscle cell and ending with cross-bridge formation.
- Which pathway is used to produce ATP in cardiac cells?
- Compare and contrast the contraction of a skeletal muscle cell with that of a cardiac muscle cell by comparing and contrasting:
- How the basic heartbeat is controlled vs. how skeletal muscle contraction is initiated.
- How the contraction of multiple neighboring muscle cells is coordinated and synchronized within cardiac muscle vs. within skeletal muscle.
- The source of the calcium ions required to promote and sustain muscle contraction in a cardiac muscle cell vs. a skeletal muscle cell.
- Compare and contrast the contraction of a smooth muscle cell with that of a cardiac muscle cell.
VIII. Describe the functional significance of self-excitatory cardiac muscle cells.
- Describe the difference in the excitation of a pacemaker cell with that of other cardiac muscle fiber cells
Part 4: Smooth Muscle Tissue
Smooth muscle (Figure 14.11), so named because the cells do not have striations, is present in the walls of hollow organs like the urinary bladder, uterus, stomach, intestines, in the walls of passageways, such as the arteries and veins of the circulatory system, and the tracts of the respiratory, urinary, and reproductive systems. Smooth muscle is also present in the eyes, where it functions to change the size of the iris and alter the shape of the lens. It is also present in the skin where it causes hair to stand erect in response to cold temperature or fear.
Smooth muscle fibers are spindle-shaped (wide in the middle and tapered at both ends, somewhat like a football) and have a single nucleus; they range from about 30 to 200 μm (thousands of times shorter than skeletal muscle fibers), and they produce their own connective tissue, endomysium. Although they do not have striations and sarcomeres, smooth muscle fibers do have thick and thin filaments composed of myosin and actin contractile proteins. These thin filaments are anchored by dense bodies. A dense body is analogous to the Z-discs of skeletal and cardiac muscle fibers and is tethered, or fastened, to the sarcolemma. Calcium ions are supplied by the sarcoplasmic reticulum (SR) in the fibers and by sequestration from the extracellular fluid through membrane indentations called caveolae.
Because smooth muscle cells do not contain troponin, cross-bridge formation is regulated not by the troponin-tropomyosin complex but instead by the regulatory protein calmodulin. In a smooth muscle fiber, external calcium ions passing through opened calcium channels in the sarcolemma, as well as additional Ca2+ released from SR, bind to calmodulin. The Ca2+-calmodulin complex then activates an enzyme called myosin (light chain) kinase, which, in turn, activates the myosin heads by phosphorylating them (converting ATP to ADP and Pi, with the Pi attaching to the head). The myosin heads can then attach to actin-binding sites and pull on the thin filaments. The thin filaments are anchored to the dense bodies, which also have cord-like intermediate filaments attached to them. In fact, intermediate filaments appear as a network throughout the sarcoplasm and are connected to each other through dense bodies. Thus, as the thin filaments slide past the thick filaments, they pull on the dense bodies, which in turn pull on the network of intermediate filaments throughout the sarcoplasm. This arrangement causes the entire muscle fiber to contract in a manner that sees its ends being pulled toward the center, causing the midsection to bulge inward, like a corkscrew (Figure 14.12).
Although smooth muscle contraction relies on the presence of calcium ions, smooth muscle fibers have a much smaller diameter than skeletal muscle cells. Smooth muscle fibers have a limited calcium-storing SR but have calcium channels in the sarcolemma (similar to cardiac muscle fibers) that open during the action potential along the sarcolemma. The influx of extracellular calcium ions, which diffuse into the sarcoplasm to reach the calmodulin, accounts for most of the Ca2+ that triggers the contraction of a smooth muscle cell.
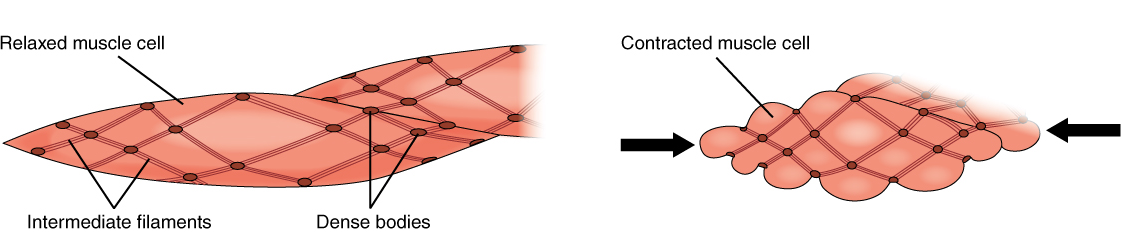
Muscle contraction continues until ATP-dependent calcium pumps actively transport calcium ions back into the SR and out of the cell. However, a low concentration of calcium remains in the sarcoplasm to maintain muscle tone. This remaining calcium keeps the muscle slightly contracted, which is important in certain tracts and around blood vessels.
Because most smooth muscles must function for long periods without rest, their power output is relatively low, but contractions can continue without using large amounts of energy. Some smooth muscle can also maintain contractions even as Ca2+ is removed and myosin kinase is inactivated/dephosphorylated. This can happen because a subset of cross-bridges between myosin heads and actin, called latch-bridges, keep the thick and thin filaments linked together for a prolonged period and without the need for ATP. This allows for the maintaining of muscle “tone” in smooth muscle that lines arterioles and other visceral organs with very little energy expenditure.
Smooth muscle is not under voluntary control; thus, it is called involuntary muscle. The triggers for smooth muscle contraction include hormones, neural stimulation by the autonomic nervous system, and local factors (e.g., localized histamine release, pH levels, etc.).
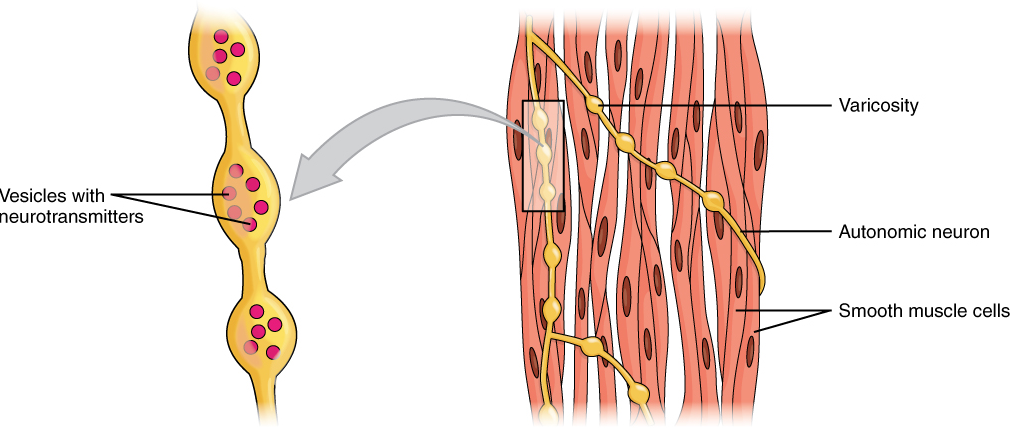
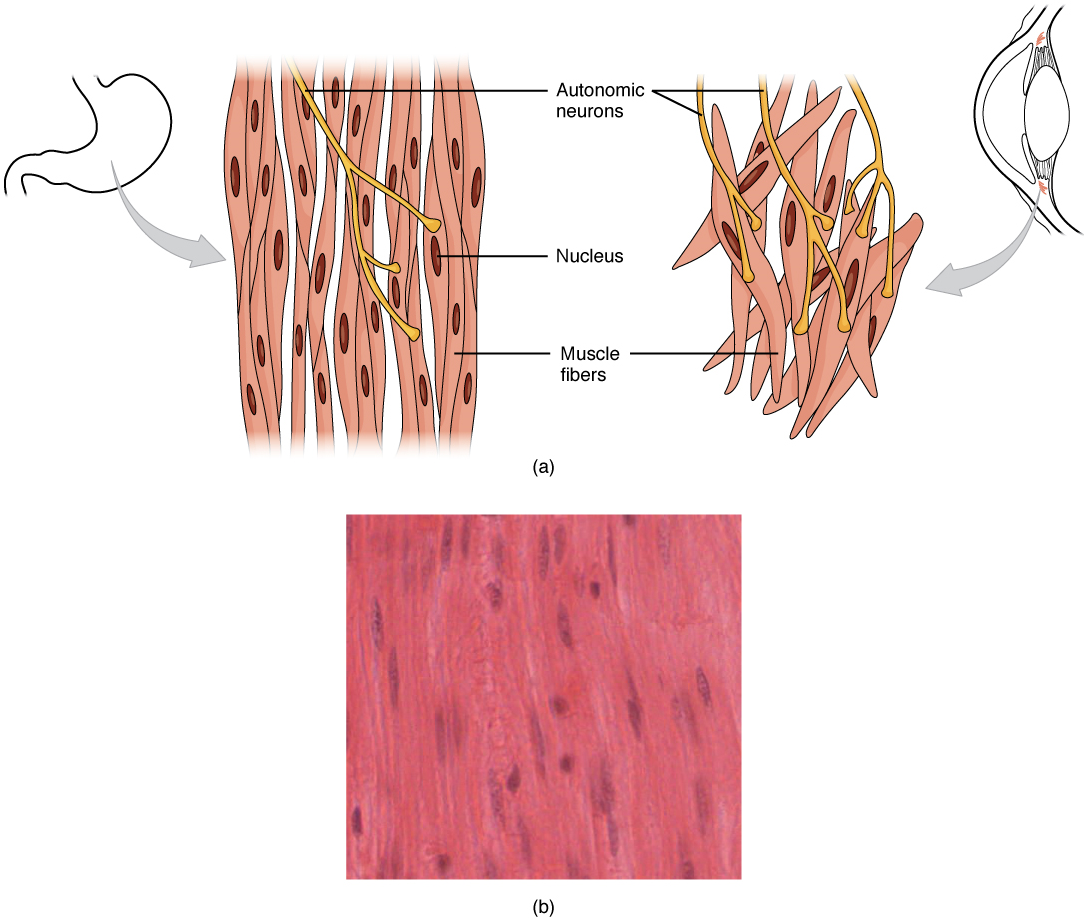
Different autonomic nerves release various neurotransmitters onto smooth muscle. For example, some nerves release acetylcholine that causes the contraction of smooth muscle around some respiratory ducts and thus the constriction of these airways. Other nerves release norepinephrine that causes the relaxation of smooth muscle and thus the widening of the airways. The same neurotransmitter can even cause opposite effects depending partly on the tissue, where it acts, and/or the variant of the neurotransmitter receptor on target cells. Although norepinephrine causes the relaxation of smooth muscle and thus the widening of some airways, it also causes the contraction of smooth muscle and thus the constriction of most blood vessels. Autonomic neurons innervating smooth muscle release their neurotransmitters from swellings along their axons, called varicosities, that tend to result in less specific localization of the released neurotransmitter than at a neuromuscular junction (Figure 14.13).
Several hormones also affect the activity of smooth muscle, either by encouraging contraction or relaxation. For example, in the digestive system, cholecystokinin induces the relaxation of the smooth muscle around the hepatopancreatic sphincter, causing it to open. Conversely, gastrin stimulates the contraction of smooth muscle in the stomach to enhance the churning activity of the stomach. Within the reproductive system, oxytocin stimulates uterine smooth muscle contraction to facilitate childbirth.
Smooth muscle arranged in layers around a hollow organ generally produces slow, steady contractions known as peristalsis that allow substances, such as food in the digestive tract, to move through the body. One layer of smooth muscle is parallel to the longitudinal axis of the lumen, and the other layer is wrapped around the lumen in a circular fashion. A third layer of longitudinal muscle (ureters) or obliquely arranged muscle (stomach) is present in some organs. This action and arrangement of smooth muscle layers causes mixing and/or unidirectional propulsion of materials through the lumen. The movement of substances through lumens by peristalsis occurs in multiple organs from different organ systems (uterus, urinary bladder, esophagus, stomach, small and large intestines) and ducts (ureters, uterine tubes, vas deferens, bile ducts).
In summary, smooth muscle is found throughout the body around various organs and tracts. Smooth muscle cells have a single nucleus and are spindle-shaped. Smooth muscle cells are nonstriated, but their sarcoplasm is filled with actin and myosin, along with dense bodies in the sarcolemma to anchor both thin filaments as well as a network of intermediate filaments, which during contraction, are together involved in pulling the sarcolemma toward the fiber’s middle, shortening it in the process.
Test Your Knowledge
IX. Describe the microscopic anatomy of smooth muscle.
- Describe the structure, location within a smooth muscle cell, and the function of each of the following:
- Caveolae
- Sarcoplasmic reticulum
- Myofilaments
- Intermediate filaments
- Dense bodies
X. Explain the mechanism of contraction and relaxation in smooth muscle.
- Describe the location and function within a smooth muscle cell of each of the following:
- Calcium ions
- Calmodulin
- Dense bodies
- Gap junctions
- Calcium ion pumps
- Describe in detail how a smooth muscle contracts by describing the events that occur within the smooth muscle from the generation of an action potential in one smooth muscle cell to contraction of the entire smooth muscle.
- Compare and contrast the contraction of a skeletal muscle cell with that of a smooth muscle cell by comparing and contrasting:
- The intracellular location and function of calmodulin vs. troponin and tropomyosin
- How the opening of calcium channels is controlled in a smooth muscle cell vs. in a skeletal muscle cell
- The source of the calcium ions required to promote and sustain muscle contraction in a smooth muscle cell vs. a skeletal muscle cell
- The types of chemicals normally used to trigger the contraction in a smooth muscle cell vs. in a skeletal muscle cell
XI. Describe the neural and hormonal factors that regulate the contraction of smooth muscle.
- For each of the following neurotransmitters, describe their effect and mechanism of action on a smooth muscle cell (i.e., contraction or relaxation):
- Acetylcholine, on a smooth muscle cell in the wall of a respiratory duct
- Norepinephrine, on a smooth muscle cell in the wall of a respiratory duct
- Norepinephrine, on a smooth muscle cell in the wall of a blood vessel
- Provide examples of conditions that would result in an increase in [Ca2+] and therefore stimulate smooth muscle contraction.
- For each of the following hormones, describe their effect, mechanism of action, and physiological significance on a smooth muscle cell (i.e., contraction or relaxation):
- Cholecystokinin, on the smooth muscle around the hepatopancreatic sphincter
- Gastrin, on the smooth muscle in the stomach wall
- Oxytocin, on the smooth muscle in the uterine wall
XII. Define the process and anatomical basis of peristalsis.
- Define the term “peristalsis.”
- Describe how sheets of smooth muscle are arranged in the wall of a hollow organ or vessel to allow peristalsis.
- Describe the function of peristalsis in the human body.
Practice
For the following question, click on the correct answer choice.
For the following activity, drag the structures to the correct empty boxes on the figures.
For the following questions, click on the correct answer choice.
For the passage below, drag and drop the correct terms to the empty boxes.
For the following questions, click the correct answer choice.
Image Descriptions
Figure 14.3 image description: This multi-sequence image describes the events of a muscular contraction. The diagram labels read (from top to down): an action potential arrives at a neuromuscular junction; ACh is released, binds to receptors, and opens sodium ion channels, leading to an action potential in sarcolemma; action potential travels along the T-tubules; calcium is released from the sarcoplasmic reticulum; calcium binds to troponin; thick and thin filament interaction leads to muscle contraction; and muscle shortens and produces tension. [Return to image.]
Figure 14.4 image description: This multi-sequence image describes the events reversing a muscular contraction (i.e., relaxation). The diagram labels read (from top to bottom): calcium is reabsorbed into sarcoplasmic reticulum beginning relaxation cycle; ATP is required; thick and thin filament interaction relaxes; and muscle lengthens and relaxes. [Return to image.]
Synapse between the axon terminal of a motor neuron and the section of the membrane of a muscle fiber with receptors for the acetylcholine released by the terminal.
Change in voltage of a cell membrane in response to a stimulus that results in transmission of an electrical signal; unique to neurons and muscle fibres.
Specialized smooth endoplasmic reticulum, which stores, releases, and retrieves Ca++.
An important neurotransmitter.
A channel protein (facilitated diffusion) that is activated (opens) when a molecule (such as a neurotransmitter) binds to it.
Change in a cell membrane potential from rest toward zero.
Ion channel that opens because of a change in the charge distributed across the membrane where it is located.
Long, cylindrical organelle that runs parallel within the muscle fiber and contains the sarcomeres.
Regulatory protein that covers myosin-binding sites to prevent actin from binding to myosin.
Regulatory protein that binds to actin, tropomyosin, and calcium.
Stiffening of the muscles and joints shortly after death due to a lack of ATP availability for breaking cross-bridges.
The default tension in a resting muscle that will optimize contraction ability.
A metabolic byproduct of cellular respiration in the absence of oxygen. It is ultimately converted back into glucose by the liver.
A multinucleate cell formed by the fusion of multiple cells or the division of nuclei.
A type of anchoring junction found between adjacent epithelial cells.
Ability to depolarize without prior nervous stimulation. This ability is a characteristic of cardiac muscle cells.
Interactions between actin and myosin filaments in smooth muscle that differ from a cross-bridge in striated muscles that have sarcomeres. The latch state is a prolonged interaction that slowly dissociates and is not ATP-dependent.
A rhythmic contraction of smooth muscle layers that line tubes, such as the esophagus, and propel substances forward.

