John and Marcia Price College of Engineering
33 Higher Wall Shear Stress in AVFs Reduces Neointimal Hyperplasia Compared to AVGs: Insights from Porcine Models
Nicholas Thomson
Faculty Mentor: Yan-Ting Shiu (Biomedical Engineering, University of Utah)
Introduction
Chronic Kidney Disease (CKD) affects approximately 15% of people in the United States, with End-Stage Kidney Disease (ESKD) representing the final stage of the condition, where the kidneys can no longer filter blood effectively [1]. At this stage, patients require renal replacement therapy to sustain life, either through a kidney transplant or hemodialysis [2]. While kidney transplants are the most effective long-term solution, their availability is severely limited, with wait times often exceeding four years [2]. Consequently, hemodialysis remains the most accessible and widely used treatment for ESKD patients due to its efficacy and availability [2].
For hemodialysis to function optimally, it requires vascular access capable of sustaining the high blood flow rates necessary for effective blood filtration by an external dialyzer. The two primary types of vascular access are arteriovenous fistulas (AVFs) and arteriovenous grafts (AVGs). AVFs are created by surgically connecting an artery to a vein, allowing the vein to enlarge and strengthen for increased blood flow. They are generally preferred due to their higher long-term success rates and fewer complications compared to AVGs, which use synthetic conduits [3]. However, both AVFs and AVGs are prone to failure due to venous stenosis, primarily caused by neointimal hyperplasia (NH), which thickens the vessel wall, reducing the lumen size and compromising blood flow [4].
Despite significant research, the biomechanical factors contributing to NH formation in AVFs and AVGs remain poorly understood, particularly the role of hemodynamic parameters like wall shear stress (WSS). The gap in understanding lies in the specific role that WSS plays in influencing NH formation, and whether these differences can account for the superior outcomes seen in AVFs. Current understanding of NH formation in vascular access settings suggests that both biological and mechanical factors are involved. Biologically, NH formation is driven by vascular smooth muscle cell (VSMC) proliferation and extracellular matrix (ECM) deposition, processes often triggered by inflammation and vascular injury [5]. Mechanically, disturbed blood flow patterns, characterized by low WSS and high oscillatory shear index (OSI), promote NH in AVGs, whereas regions with higher and more stable WSS are associated with better outcomes in AVFs [5], [6], [7]. Computational models, like those using Computational Fluid Dynamics (CFD), enable detailed simulations of these hemodynamic conditions, providing insights into how WSS and OSI affect NH progression in patient-specific vascular geometries [6], [7].
This study seeks to further address this gap by investigating how early hemodynamic differences between AVFs and AVGs influence NH formation, focusing specifically on WSS. The hypothesis is that AVFs, which experience higher WSS, exhibit reduced NH compared to AVGs. This hypothesis will be explored using porcine models and validated through CFD simulations and statistical analysis to ensure model accuracy. By integrating findings from these simulations with biological insights, the study aims to clarify the biomechanical influences on NH, ultimately contributing to improved vascular access management and clinical outcomes for hemodialysis patients.
Background
Neointimal hyperplasia is a pathological process characterized by the proliferation of VSMCs and the accumulation of ECM components within the intima of blood vessels following injury. This response is primarily triggered by the initial vascular trauma, activating VSMCs to migrate from the medial to the intimal layer and proliferate [4]. NH formation plays a critical role in restenosis, or the re-narrowing of blood vessels, after interventions such as vascular grafting and percutaneous coronary interventions [8]. The mechanisms underlying NH are complex and multifactorial, involving an interplay of cellular responses, inflammation, and ECM remodeling, which together contribute to structural changes in the vessel wall [9].
Biological studies reveal that the early response to vascular injury includes the release of growth factors, such as platelet-derived growth factor, which promotes VSMC proliferation and migration [10]. The proliferation of VSMCs is central to NH formation, as these cells lay down ECM components that structurally reinforce the neointima, although excessive proliferation leads to vessel narrowing. Research indicates that targeting VSMC behavior can mitigate NH; for example, metformin has been shown to reduce VSMC proliferation, thus decreasing NH in insulin-resistant models [11]. Inflammatory cells, particularly macrophages, also play a key role in NH development by releasing cytokines and matrix metalloproteinases (MMPs) that promote VSMC migration and differentiation [12], [13]. This interplay between VSMCs and inflammatory mediators highlights the need for therapeutic strategies addressing both cellular proliferation and inflammatory responses.
The role of ECM remodeling is another essential factor in NH progression. ECM components like collagen are deposited by activated VSMCs, adding structural integrity to the neointima while contributing to lumen narrowing. Studies show that the balance between ECM deposition and degradation is pivotal in determining NH extent. For instance, inhibiting MMPs can slow ECM deposition, reducing NH [14]. Additionally, advancements in drug-eluting stents and gene therapy have offered new methods for curbing NH by delivering antiproliferative agents directly to the site of vascular injury [15]. Drug-eluting stents, in particular, have been effective in reducing NH compared to bare-metal stents, and gene therapy targeting microRNAs has shown promise in modulating VSMC behavior to prevent NH [16].
Computational methods, particularly CFD, provide valuable tools for understanding the hemodynamic factors associated with NH in vascular access settings. CFD modeling enables detailed simulations of flow patterns and forces within vascular structures, offering insights into how hemodynamic conditions influence NH formation [6]. Studies utilizing CFD in AVFs and AVGs have shown that disturbed blood flow patterns, characterized by low WSS and high OSI, are associated with increased NH in AVGs, while regions with stable, high WSS correlate with better AVF outcomes [7,17]. By applying CFD to patient-specific vascular geometries, researchers can simulate the hemodynamic environment and evaluate potential interventions in a non-invasive manner. These models are especially relevant to this study, as they enable an in-depth analysis of WSS in AVFs versus AVGs, addressing a critical gap in understanding the biomechanical factors that influence NH formation in vascular access.
Methods
Materials
This study used six female Yorkshire cross domestic pigs, selected for their anatomical and physiological similarities to human vascular systems, making them suitable models for hemodynamic analysis in vascular access research [8]. All procedures were approved under Institutional Animal Care and Use Committee (IACUC). The pigs were divided into two groups: three for the AVF model and three for the AVG model. AVFs were surgically created by connecting the common carotid artery to the ipsilateral external jugular vein. For the AVG group, a 7-cm expanded polytetrafluoroethylene graft with a 6-mm internal diameter was surgically implanted between the carotid artery and the external jugular vein. The animals were monitored post-surgery to ensure consistent recovery conditions across both groups, minimizing potential variations in hemodynamic response [9].
Imaging and Data Acquisition
Two weeks post-surgery, magnetic resonance imaging (MRI) was conducted using a 3T Siemens Magnetom Prisma scanner to capture detailed images of the vascular structures, enabling a comprehensive assessment of the hemodynamic environment. Imaging protocols included 2D time-of-flight imaging for high-resolution visualization of vascular structures, 3D black blood MRI to define the vessel lumen and wall, and 2D phase-contrast MRI to measure blood flow velocities at specific locations within the AVFs and AVGs [10]. These imaging techniques provided detailed geometric and flow profiles essential for subsequent computational modeling.
Computational Fluid Dynamics (CFD)
Following image acquisition, computational methods were used to reconstruct the 3D lumen geometry of the AVFs and AVGs. Segmentation software (Amira, version 2022.1) was used to create detailed vessel representations. High-resolution meshes were generated in Ansys ICEM CFD (version 2022 R2). Each AVF model contained approximately 1.5 million elements, while the AVG models included around 3 million elements, ensuring the spatial resolution necessary for accurate CFD simulations [11]. Ansys Fluent (version 2022) was then used to perform transient simulations across three cardiac cycles, with the third cycle analyzed to obtain steady-state flow parameters. Velocity profiles extracted from the MRI data were applied as inlet conditions, and additional flow extensions, generated using Vascular Modeling Toolkit (VMTK, version 1.5), ensured realistic boundary conditions for the computational model [12].
Data Processing and Analysis
The CFD simulation data were processed in Tecplot (version 2023 R1) to extract critical hemodynamic parameters, including wall shear stress (WSS), oscillatory shear index (OSI), blood velocity, and vorticity. Statistical analysis was performed using GraphPad Prism (version 2024), applying paired t-tests to evaluate significant differences between AVFs and AVGs. Model accuracy was validated by comparing simulated flow parameters with MRI-measured velocities. This analysis focused on quantifying the relationship between WSS and other key hemodynamic factors, supporting our hypothesis that higher WSS in AVFs reduces NH formation.
Results
Wall shear stress analysis demonstrated a significantly higher WSS in AVFs compared to AVGs, particularly in the proximal vein (PV). AVFs exhibited an average WSS of 382.8 ± 82.2 dyne/cm² in the PV, compared to 332.9 ± 108.4 dyne/cm² in AVGs (Table 1). These differences are visualized in the heat maps (Figure 1), which highlight the greater and more stable shear forces present in AVFs. Higher WSS is associated with reduced neointimal hyperplasia (NH), as it improves endothelial cell alignment and reduces vascular injury [5], [6].
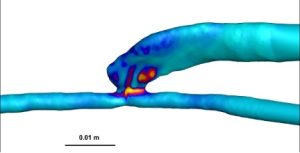
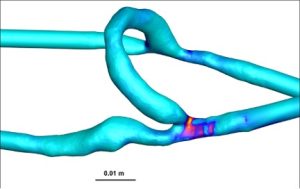
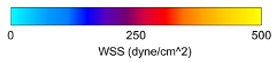
Figure 1: Representative heat maps of WSS in AVFs (left) and AVGs (right). AVFs exhibit significantly higher WSS values across the proximal vein.
| Parameter | AVF |
AVG
|
| CSA (mm²) | 28.3 ± 4.2 | 9.5 ± 0.4 |
| Flow Rate (mL/min) | 1122.4 ± 47.8 | 862.7 ± 87.2 |
| Vorticity (rotations/s) | 1880.6 ± 206 | 1174.3 ± 179.9 |
| Velocity (m/s) | 1.2 ± 0.2 | 1.2 ± 0.1 |
| WSS (dyne/cm²) | 382.8 ± 82.2 | 332.9 ± 108.4 |
Table 1: Comparison of early hemodynamic differences in the proximal vein between AVFs and AVGs, including CSA, velocity, and WSS.
Velocity analysis revealed similar mean velocities in the proximal vein between AVFs and AVGs, both averaging 1.2 m/s (Table 1). However, streamline visualizations (Figure 2) showed that AVFs had more laminar and organized flow patterns, whereas AVGs demonstrated disturbed flow. These laminar patterns in AVFs are hypothesized to provide a protective hemodynamic environment that reduces NH development [6].
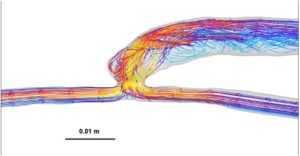
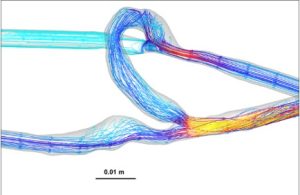
Figure 2: Representative heat maps of velocity distribution with streamline overlays for AVFs (left) and AVGs (right). AVFs show laminar flow, while AVGs exhibit disturbed flow patterns.
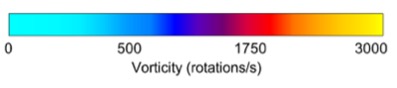
Vorticity, a measure of rotational flow, was significantly higher in AVFs compared to AVGs, with values of 1880 ± 206 rotations/s in the PV for AVFs versus 1174 ± 180 rotations/s for AVGs (Table 1). This increased vorticity suggests enhanced blood mixing and wall stimulation in AVFs, potentially contributing to reduced NH formation. Heat maps (Figure 3) further illustrate these differences, highlighting regions of greater rotational energy in AVFs.
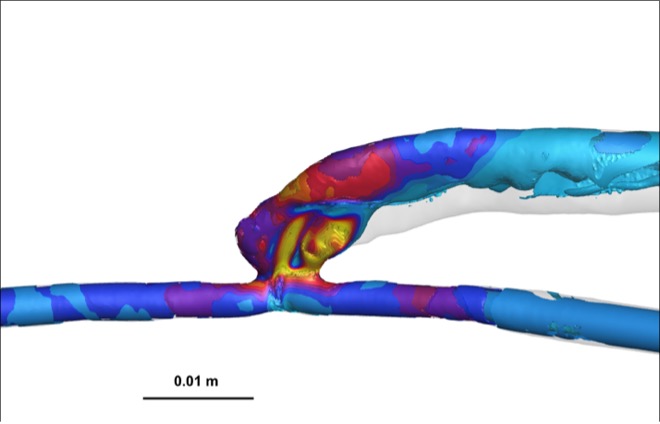
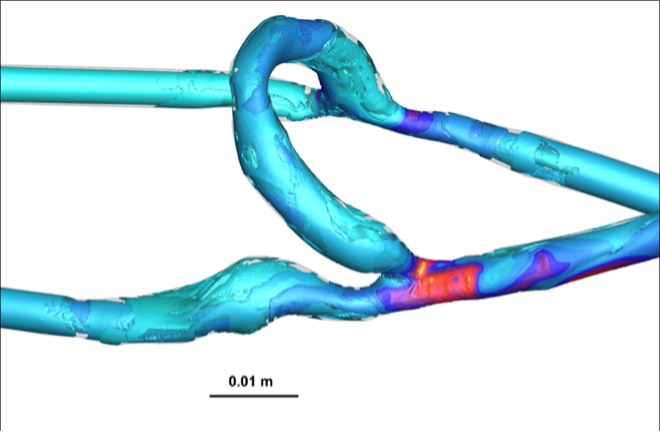
The OSI, indicative of disturbed flow, was significantly lower in AVFs compared to AVGs (Figure 4). OSI values in AVFs were approximately 0.15, whereas AVGs exhibited OSI values closer to 0.25. Lower OSI reflects more stable flow conditions, which are protective against NH formation [7].
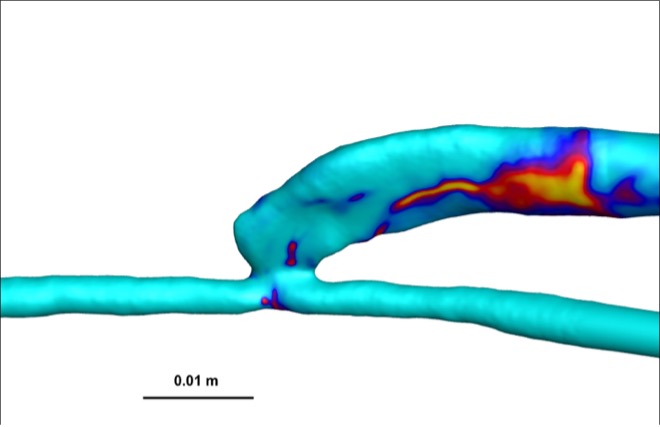
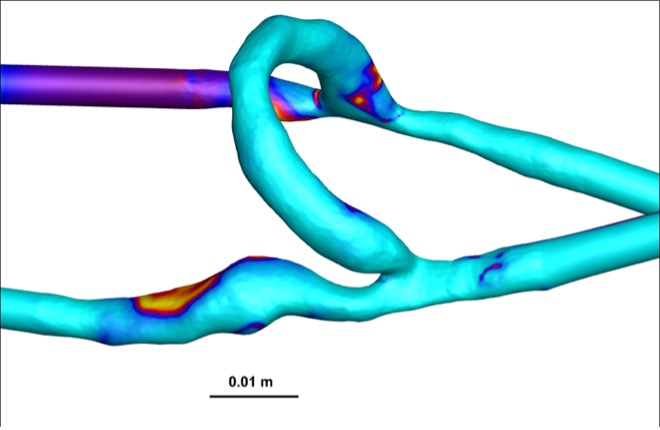
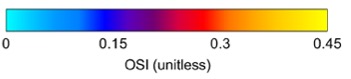
Figure 4: Heat maps of OSI for AVFs (top) and AVGs (bottom). AVFs display lower OSI values, indicative of more stable flow.
In the proximal artery, AVFs exhibited larger cross-sectional area (CSA) and higher velocity and WSS compared to AVGs (Table 2). These findings complement the proximal vein analysis, further reinforcing the biomechanical advantages of AVFs across vascular regions.
Together, these results emphasize that AVFs offer a hemodynamically advantageous environment, which supports their long-term functionality and clinical success in hemodialysis.
| Parameter | AVF | AVG |
| CSA (mm²) | 17.1 ± 1.4 | 12.1 ± 2.0 |
| Flow Rate (mL/min) | 722.1 ± 127.9 | 320.9 ± 76.3 |
| Vorticity (rotations/s) | 662.1 ± 116 | 628.1 ± 134.5 |
| Velocity (m/s) | 0.62 ± 0.1 | 0.50 ± 0.1 |
| WSS (dyne/cm²) | 81.5 ± 28.2 | 34.0 ± 12.7 |
Table 2: Comparison of early hemodynamic differences in the proximal artery between AVFs and AVGs, showing higher flow rates, vorticity, and WSS in AVFs.
Discussion
This study aimed to evaluate how hemodynamic differences, particularly wall shear stress (WSS), influence neointimal hyperplasia (NH) formation in arteriovenous fistulas (AVFs) compared to arteriovenous grafts (AVGs). Using porcine models and computational fluid dynamics (CFD) simulations, we tested the hypothesis that AVFs exhibit higher WSS and lower oscillatory shear index (OSI), leading to reduced NH formation. The findings confirmed this hypothesis, demonstrating that AVFs maintain significantly higher WSS in the proximal vein (382.8 ± 82.2 dyne/cm²) compared to AVGs (332.9 ± 108.4 dyne/cm²). The increased shear stress likely contributes to improved endothelial function and lower NH progression, reinforcing why AVFs remain the preferred vascular access method for hemodialysis patients. These results suggest that WSS plays a central role in shaping vascular remodeling outcomes and could be a critical factor in optimizing graft designs.
The relationship between WSS and NH is further emphasized by the differences observed in OSI and vorticity. AVFs exhibited lower OSI (0.15) compared to AVGs (0.25), which suggests a more stable and protective flow environment. Higher OSI has been linked to endothelial dysfunction, increased inflammation, and greater NH progression, supporting the idea that AVFs maintain a more favorable biomechanical environment. Additionally, vorticity was significantly higher in AVFs (1880 ± 206 rotations/s) compared to AVGs (1174 ± 180 rotations/s), indicating enhanced blood mixing and wall stimulation. The dynamic nature of flow in AVFs may contribute to a protective effect against NH, as high vorticity has been associated with improved endothelial adaptation and reduced vessel narrowing. Together, these findings reinforce the biomechanical advantage of AVFs and highlight potential areas for improving AVGs.
These results align with previous studies that have examined the impact of hemodynamics on vascular remodeling. Prior work has shown that AVFs consistently exhibit higher WSS, often exceeding 350 dyne/cm² in mature fistulas, a threshold associated with improved long-term patency rates [6]. Similarly, previous studies have found that AVGs, which experience lower and more oscillatory shear stress, are more prone to NH and stenosis [7]. The consistency between these findings and the present study supports the idea that AVF success is largely influenced by its hemodynamic profile. The observed differences in vorticity also complement prior computational models that suggest rotational flow enhances endothelial adaptation, a feature that appears to be more prominent in AVFs due to their natural flow dynamics. While these findings add to the growing body of literature on vascular access biomechanics, they also point toward potential interventions to mitigate NH formation in AVGs.
A key limitation of this study is the small sample size (n=6), which restricts statistical power and generalizability. Additionally, while porcine models closely approximate human vascular physiology, interspecies differences may affect the direct clinical translation of these results. The study also focuses primarily on mechanical factors such as WSS and OSI, without directly investigating the biological pathways involved in NH formation, such as inflammation, endothelial dysfunction, or smooth muscle cell proliferation. Integrating biological markers with computational models in future studies would provide a more comprehensive understanding of the mechanobiology underlying vascular access outcomes.
Despite these limitations, the findings have important clinical implications. The confirmation that higher WSS in AVFs is associated with reduced NH suggests that modifications to AVG design could enhance their longevity and functionality. Potential interventions could include altering graft geometry to increase localized WSS, developing bioactive coatings that promote endothelial adaptation, or incorporating patient-specific CFD modeling to optimize vascular access planning. These strategies could help mitigate NH formation and extend the viability of AVGs, particularly for patients who are not candidates for AVFs due to vascular limitations.
Future research should address the limitations of this study by increasing the sample size, incorporating long-term follow-up data, and integrating biological analyses such as inflammatory cytokine profiling and histological evaluation. Additionally, expanding CFD simulations to account for patient-specific vascular geometries could provide deeper insights into optimizing vascular access selection and surgical planning. By bridging computational modeling with clinical application, future studies can refine strategies to improve vascular access outcomes and reduce complications related to NH progression.
Ultimately, these findings contribute to the broader effort to enhance vascular access strategies for hemodialysis patients, a population that faces high morbidity and mortality due to access failure. By demonstrating that higher WSS in AVFs correlates with reduced NH, this study reinforces the long-standing preference for AVFs and provides a foundation for improving AVGs. Further exploration of hemodynamic optimization could lead to more durable access solutions, improving patient outcomes and reducing the healthcare burden associated with vascular access complications.
References
[1] Centers for Disease Control and Prevention, “Chronic Kidney Disease Basics,” CDC, Feb. 28, 2022. [Online]. Available: www.cdc.gov/kidneydisease/basics.html
[2] J. S. Thurlow, M. Joshi, G. Yan, et al., “Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy,” American Journal of Nephrology, vol. 52, no. 2, pp. 98-107, 2021.
[3] J. Kolikof, K. Peterson, and A. M. Baker, “Central Venous Catheter,” in StatPearls, Treasure Island, FL: StatPearls Publishing, Jul. 26, 2023. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK557798/
[4] T. C. Rothuizen, C. Wong, P. H. A. Quax, A. J. Van Zonneveld, T. J. Rabelink, and J. I. Rotmans, “Arteriovenous access failure: More than just intimal hyperplasia?,” Nephrology Dialysis Transplantation, vol. 28, no. 5, pp. 1085-1092, 2013.
[5] E. Rajabi-Jagahrgh, M. K. Krishnamoorthy, P. Roy-Chaudhury, et al., “Longitudinal assessment of hemodynamic endpoints in predicting arteriovenous fistula maturation,” Seminars in Dialysis, vol. 26, no. 2, pp. 203-209, 2013.
[6] Y. He, H. Northrup, H. Le, A. K. Cheung, S. A. Berceli, and Y. T. Shiu, “Medical Image- Based Computational Fluid Dynamics and Fluid-Structure Interaction Analysis in Vascular Diseases,” Frontiers in Bioengineering and Biotechnology, vol. 10, 2022.
[7] M. S. Hafeez, M. H. Eslami, R. A. Chaer, and T. H. Yuo, “Comparing post-maturation outcomes of arteriovenous grafts and fistulae,” The Journal of Vascular Access, 2023.
[8] L. Li, C. M. Terry, Y. T. Shiu, and A. K. Cheung, “Neointimal hyperplasia associated with synthetic hemodialysis grafts,” Kidney International, vol. 74, no. 10, pp. 1247–1261, 2008.
[9] M. Wang, C. Qiu, Q. Pan, Y. Yang, D. Yang, and X. Sun, “Role of nuclear receptor subfamily 1 group D member 1 in the proliferation, migration of vascular smooth muscle cell, and vascular intimal hyperplasia,” Journal of Cardiovascular Pharmacology, vol. 82, no. 3, pp. 221-228, 2023.
[10] J. Lü, J. Ji, H. Meng, D. Wang, B. Jiang, L. Liu, et al., “The protective effect and underlying mechanism of metformin on neointima formation in fructose-induced insulin-resistant rats,” Cardiovascular Diabetology, vol. 12, 2013.
[11] Q. Zhao, D. Zhou, H. You, B. Lou, Y. Zhang, Y. Tian, et al., “IFN-γ aggravates neointimal hyperplasia by inducing endoplasmic reticulum stress and apoptosis in macrophages by promoting ubiquitin-dependent liver X receptor-α degradation,” The FASEB Journal, vol. 31, no. 12, pp. 5321-5331, 2017.
[12] F. Yang, Q. Chen, M. Yang, E. Maguire, X. Yu, S. He, et al., “Macrophage-derived MMP-8 determines smooth muscle cell differentiation from adventitia stem/progenitor cells and promotes neointima hyperplasia,” Cardiovascular Research, vol. 116, no. 1, pp. 211-225, 2019.
[13] X. Shen, J. Zou, F. Li, T. Zhang, and T. Guo, “Lysophosphatidic acid enhances neointimal hyperplasia following vascular injury through modulating proliferation, autophagy, inflammation, and oxidative stress,” Molecular Medicine Reports, vol. 17, no. 1, pp. 215-222, 2018.
[14] W. Zhang, Y. Cheng, Y. Xiao, Y. Sun, Y. Lin, Q. Li, et al., “Sulfasalazine induces autophagy inhibiting neointimal hyperplasia following carotid artery injuries in mice,” Frontiers in Bioengineering and Biotechnology, vol. 11, 2023.
[15] E. Bahnson, H. Kassam, T. Moyer, W. Jiang, C. Morgan, J. Vercammen, et al., “Targeted nitric oxide delivery by supramolecular nanofibers for the prevention of restenosis after arterial injury,” Antioxidants & Redox Signaling, vol. 24, no. 8, pp. 401-418, 2016.
[16] S. Cassese, R. Byrne, T. Tada, S. Pinieck, M. Joner, T. Ibrahim, et al., “Incidence and predictors of restenosis after coronary stenting in 10,004 patients with surveillance angiography,” Heart, vol. 100, no. 2, pp. 153-159, 2013.
[17] J. Liu, Y. Peng, J. Lai, W. Gao, A. Song, and G. Zhang, “Fluid upstream shear stress of rabbit aortic stenosis inhibits neointimal hyperplasia by promoting endothelialization after balloon injury,” BMC Cardiovascular Disorders, vol. 17, no. 1, p. 35, 2017.
[18] N. R. Neyra and S. Wazir, “The evolving panorama of vascular access in the 21st century,” Frontiers in Nephrology, vol. 2, 2022.
[19] P. Roy-Chaudhury, “Endothelial Progenitor Cells, Neointimal Hyperplasia, and Hemodialysis Vascular Access Dysfunction: Novel Therapies for a Recalcitrant Clinical Problem,” Circulation, vol. 112, no. 1, pp. 3-4, 2005.
[20] M. S. MacNealy, Strategies for Empirical Research in Writing. Boston, MA
Media Attributions
- wss1 (fig. 1)
- wss2 (fig. 1)
- wss key (fig. 1)
- 137489262_vel_1
- 146879582_vel_2
- velocity key (fig. 2)
- vort 1 (fig. 3)
- vort 2 (fig. 3)
- vort key (fig. 3)
- osi 1 (fig. 4)
- osi 2 (fig. 4)
- osi key (fig. 4)

