College of Science
105 The Impact of Single Amino Acid Substitutions in the CheA Linker 3 Sequence on the Chemotactic Signal Transduction Pathway of Escherichia coli
Savannah Romney
Faculty Mentor: John Parkinson (School of Biological Sciences, University of Utah)
Abstract
Every motile cell can monitor and track its chemical environment, a behavior known as chemotaxis (1). The chemotaxis machinery of bacterial cells like Escherichia coli (E. coli) enables them to move toward beneficial chemicals (attractants) and away from harmful ones (repellents). Central to this process is signal transduction which allows cells to monitor their extracellular environment and generate an appropriate response (11). At the core of E. coli’s chemotaxis machinery lies the CheA signaling protein, often called its “central processing unit” (7). CheA is responsible for several functions of the signal transduction process, including autophosphorylation. For autophosphorylation to occur, two domains of the CheA protein, P4 and P1, must be in close proximity to interact. A short, flexible linker connecting the P3 and P4 domains of CheA, referred to as L3, plays a significant role in the movement of the P4 domain toward the P1 domain to promote interaction.
To understand the structural and functional significance of the third linker of histidine- kinase CheA, I made single amino acid substitutions at each position of the 6-residue linker. Codon changes in the cheA gene were chosen to encode amino acids with various chemical properties. I classified each mutant based on its ability to support chemotaxis and tested protein expression levels and stability by means of western blot techniques. I further characterized these mutants through Förster resonance energy transfer (FRET) assays of CheA activity in living cells. These data determined whether a mutant kinase functioned as well as the wild type, or if it had a functional defect that interfered with the communication between the chemoreceptors and CheA.
I found that 22 out of 28 of the amino acid substitutions that I created severely impaired the chemotactic behavior of the bacteria. I also concluded that all mutants had protein expression levels comparable to those of wildtype, indicating that the mutant proteins are expressed and stable in the cell. Finally, I assessed how well the serine receptor Tsr communicated with the mutant CheA proteins and determined that some CheA lesions decreased sensitivity to serine and others abrogated CheA autophosphorylation ability.
Introduction
E. coli is a model organism commonly used in molecular biology and biochemistry research because it is easy to handle in the lab and is highly diverse. It is found naturally in the digestive systems of healthy humans and animals (10). E. coli signal transduction is widely studied. Researchers have developed a current working model of the E. coli receptor system that shows how a bacterium responds to stimuli in its external environment. Each E. coli cell has clusters of chemoreceptors that sense chemicals in their extracellular environment. The protein kinase, CheA, will respond to information transmitted through the transmembrane chemoreceptors. This transduction of signals ultimately makes its way to the flagella and produces a movement that responds to external stimuli in the cell’s environment (9). Current research on this behavior aims to understand the signal transduction process on a molecular level.
At this time, the predicted structures and roles of proteins in the receptor systems are well understood (14). However, there is less certainty about the sensitivity of the receptor systems to mutational changes. Creating mutations in the receptor systems of E. coli is a current method used to identify how changes to the amino acid sequence of the protein change its conformations and functionality (7). The CheA protein has 5 domains, each joined to one another by linkers.
One linker in particular, L3, is small and possibly flexible. L3 is thought to be responsible for a movement in the P4 domain of the CheA protein that promotes a productive interaction with the P1 domain that transfers a phosphate group from the P4 domain to the P1 domain.
Background
History of E. coli in the Laboratory
The discovery of E. coli occurred in 1884 when microbiologist and pediatrician Theodor Escherich discovered the organism when studying gut health in infants. Escherich, after whom E. coli was named, was the first person to isolate and characterize the bacterium (10). Over time, researchers recognized that E. coli was a versatile bacterium ideal for laboratory use and manipulation. As time progressed, researchers began studying how E. coli interacted with its extracellular environment. Julius Adler was known as a pioneer in the field of molecular biology. In 1960, Adler discovered that E. coli sensed its external chemical environment to move in a favorable direction (2). E. coli cells move toward higher concentrations of favorable amino acids by controlling the direction of flagellar movement (4). While E. coli cells move toward “attractive” chemicals, the cells do not move linearly. Instead, these prokaryotic cells complete “random walks,” or a combination of “runs” and “tumbles.” Runs are forward swimming movements produced when the flagella of the cell rotate in a counterclockwise (CCW) direction. Tumbles interfere with run episodes and randomize the next direction of forward travel. Tumbles occur when the flagella are signaled to rotate in a clockwise (CW) direction (7). These responses to the cell’s chemical environment promote movement toward favorable extracellular environments and away from unfavorable ones. This behavior is known as chemotaxis (Refer to Figure 1).
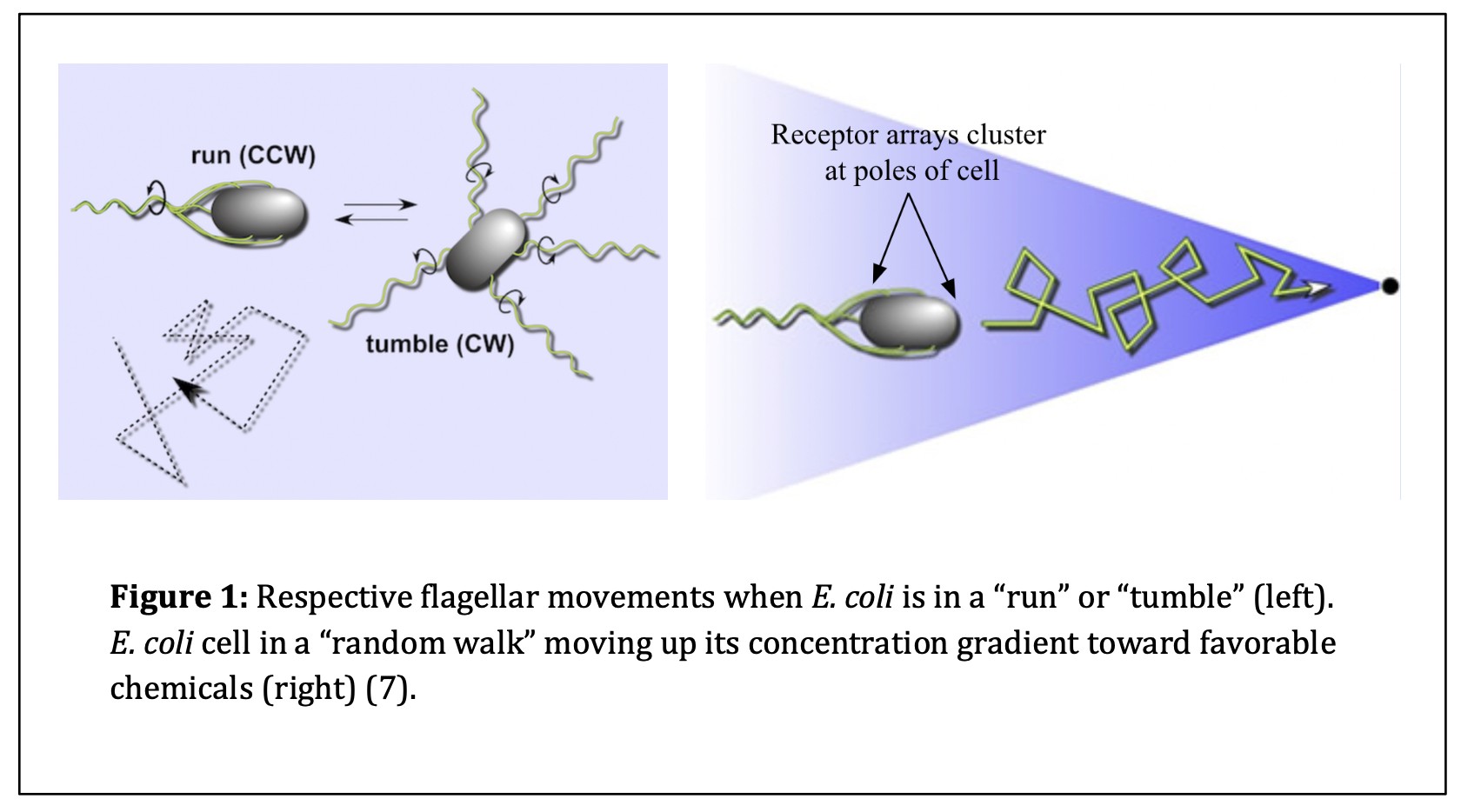
Contributing Research on E. coli Chemotaxis
Core Complex Structure
Following the discovery of bacterial chemotaxis, researchers looked at the molecular mechanisms that allow E. coli to respond to extracellular stimuli. E. coli interacts with the extracellular environment through chemoreceptors that bind specific amino acids (3). These chemoreceptors are one component of a “core complex” that connects different parts of the E. coli receptor system (14). Each core complex contains six receptor molecules arranged as two trimers, a homodimeric kinase (CheA), and two copies of a scaffolding protein (CheW) (15). Figure 2 depicts the components and interactions between these “core complexes.” Researchers learned that many core complexes are networked into large arrays that cluster at the two poles of the cell. Together, these arrays initiate transmission of stimulus signals from the transmembrane chemoreceptors to the flagellar motors of the E. coli cell to produce the appropriate locomotor response to the extracellular chemical environment (16).
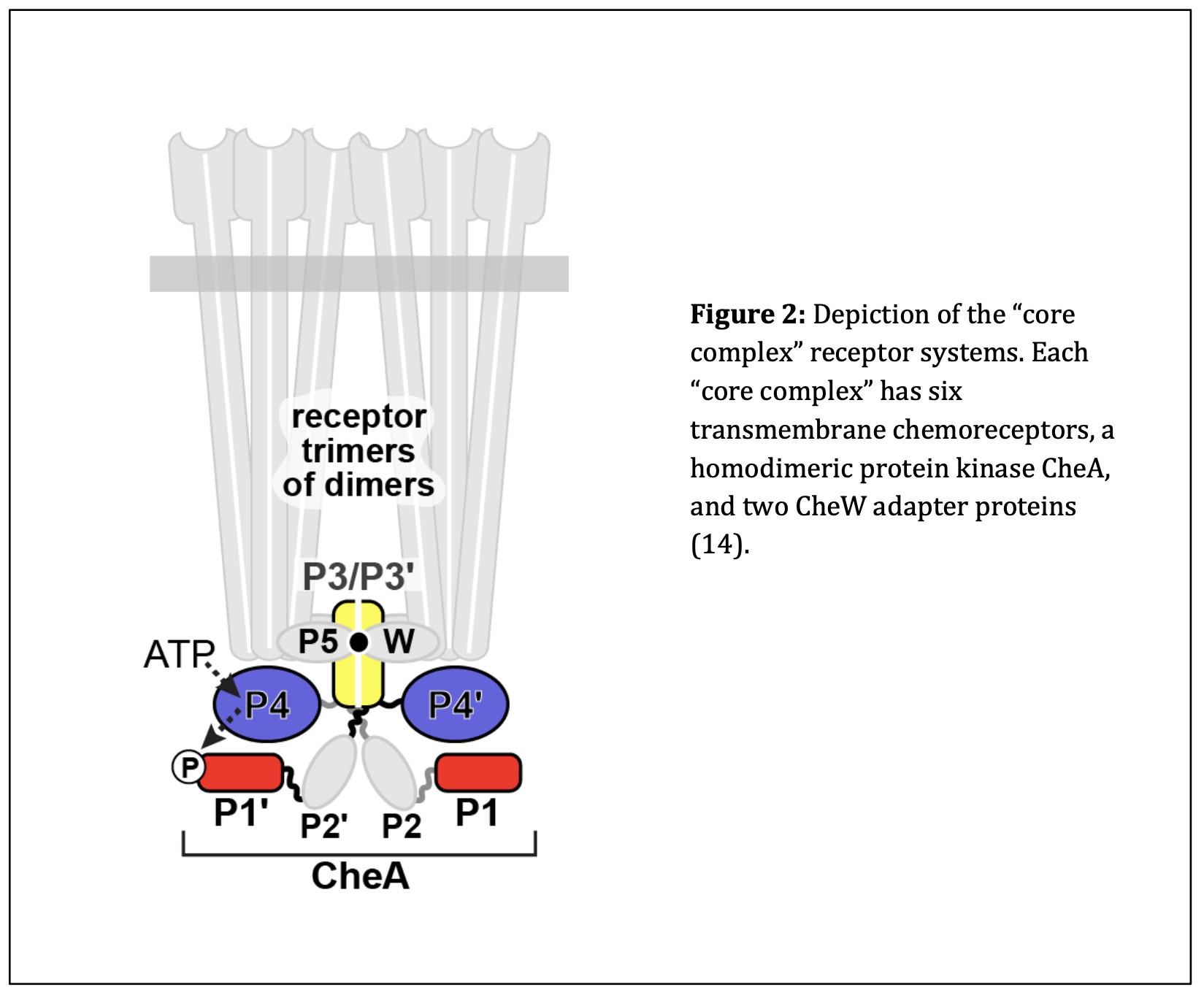
Types of Chemoreceptors
There are a variety of chemoreceptors that bind specific ligands in the E. coli receptor system. The two most abundant E. coli chemoreceptors are Tsr and Tar. Tsr chemoreceptors bind primarily serine and several other amino acids at their extracellular binding site (15). Serine is an attractant for E. coli, so when serine is detected, the cell responds accordingly by deactivating the kinase CheA. Tsr receptors are also able to identify repellents in the extracellular environment, encouraging the opposite cellular response (16). Tar receptors bind aspartate, another attractant amino acid. When aspartate is detected, the cells similarly deactivate the kinase to continue movement in the forward direction (16). While the research community knows a lot of information about the structures of these chemoreceptors, the molecular signaling mechanisms from the ligand binding site of the receptor to the terminal end of the receptor that interacts with CheA are largely unknown.
Current Findings on the Histidine Kinase, CheA
CheA Domains
CheA is the histidine protein kinase responsible for receiving signals from the transmembrane chemoreceptors and transmitting that information to the flagellar machinery to promote appropriate movement in E. coli (12). The CheA histidine protein kinase has five domains connected by short linkers (5). Each protein domain of CheA has a unique function in signal transduction. The P1 domain of CheA bears a histidine residue that is the site of CheA autophosphorylation. The P2 domain binds CheY/CheB, two auxiliary proteins involved in the signal transduction process. CheY obtains phosphoryl groups from CheA and then interacts with the flagellar motors to trigger CW reversals and cell tumbling. The P3 domain in CheA subunits self-associates to form CheA homodimers, the active form of the protein. The P4 domain binds the small molecule phosphodonor ATP and upon interaction with the P1 domain transfers a phosphate group to P1 (14). The molecular mechanisms that control interactions between the P4 and P1 domains are not well understood (7,12). The P5 domain in each CheA subunit binds to a CheW adaptor protein to assemble core signaling units and to bring CheA under chemoreceptor control. These functions can be seen in the linear model of the CheA protein domains and linkers in Figure 3.
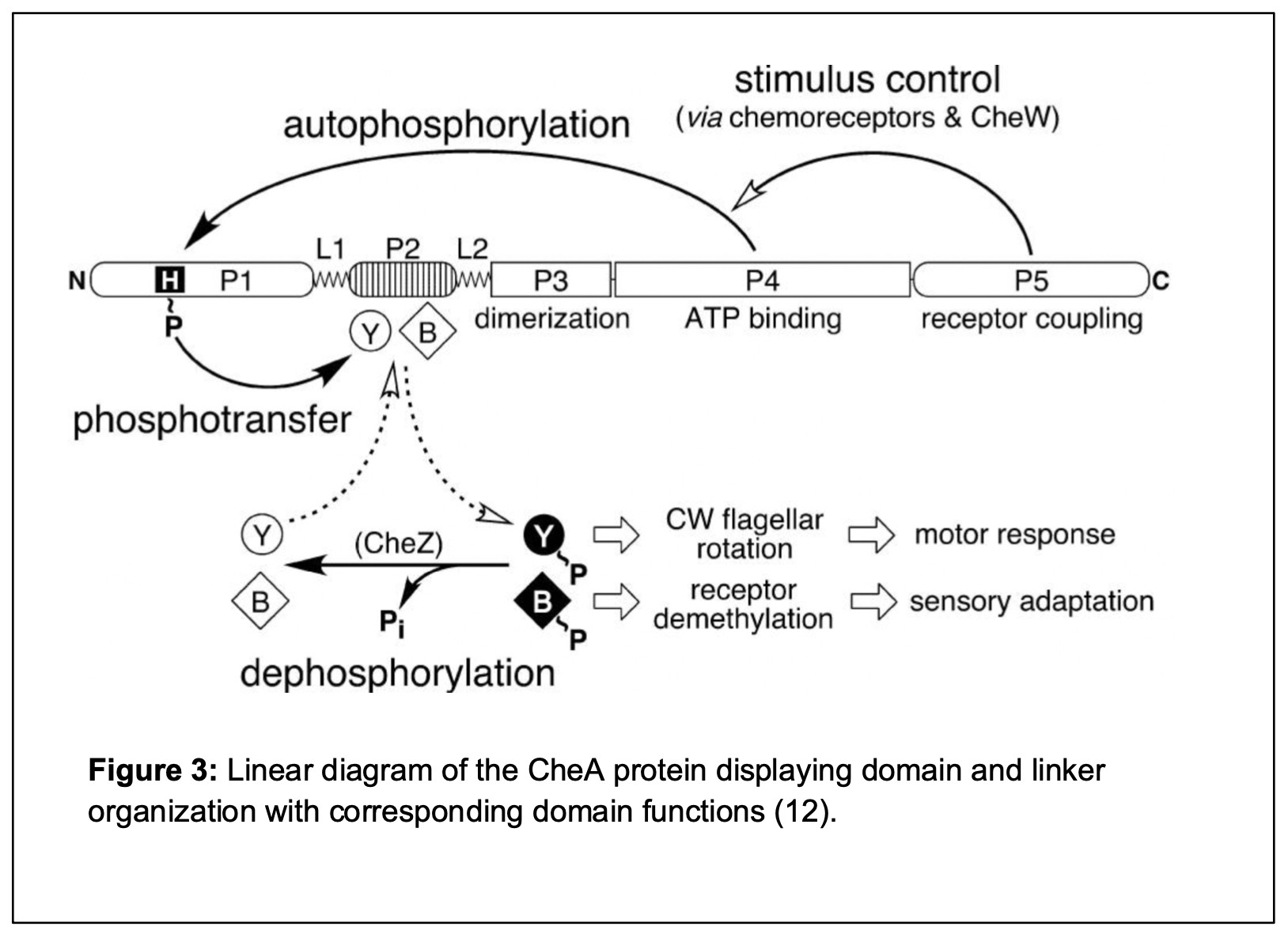
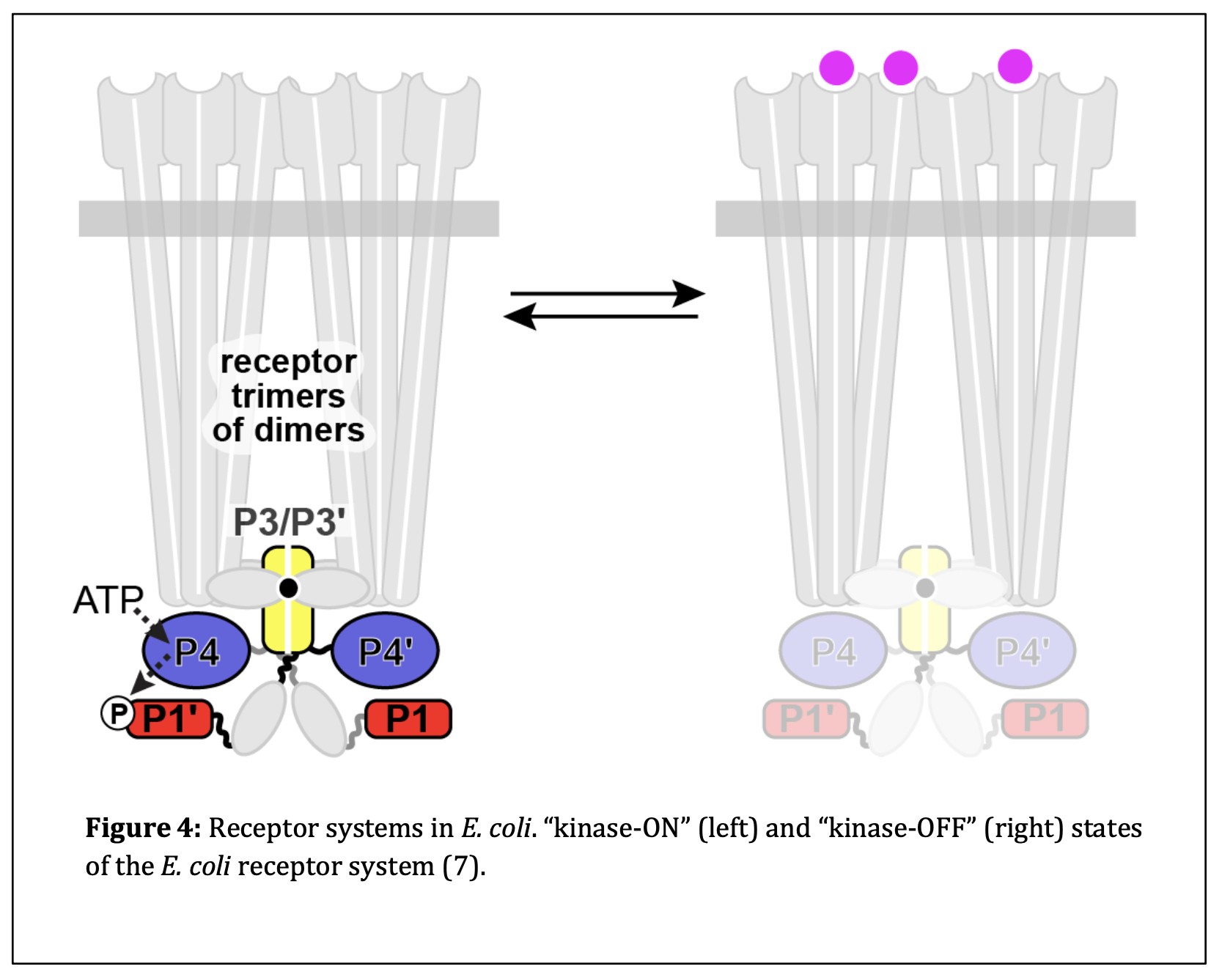
“Kinase-ON” and “Kinase-OFF” CheA States
E. coli chemoreceptors have two output states: “kinase-ON” and “kinase-OFF.” The kinase- ON state occurs when CheA is catalytically active in the absence of attractant molecules. Upon sensing an attractant, chemoreceptors switch to the kinase-OFF state and deactivate CheA, thereby promoting forward swimming of the cell (Refer to Figure 4).
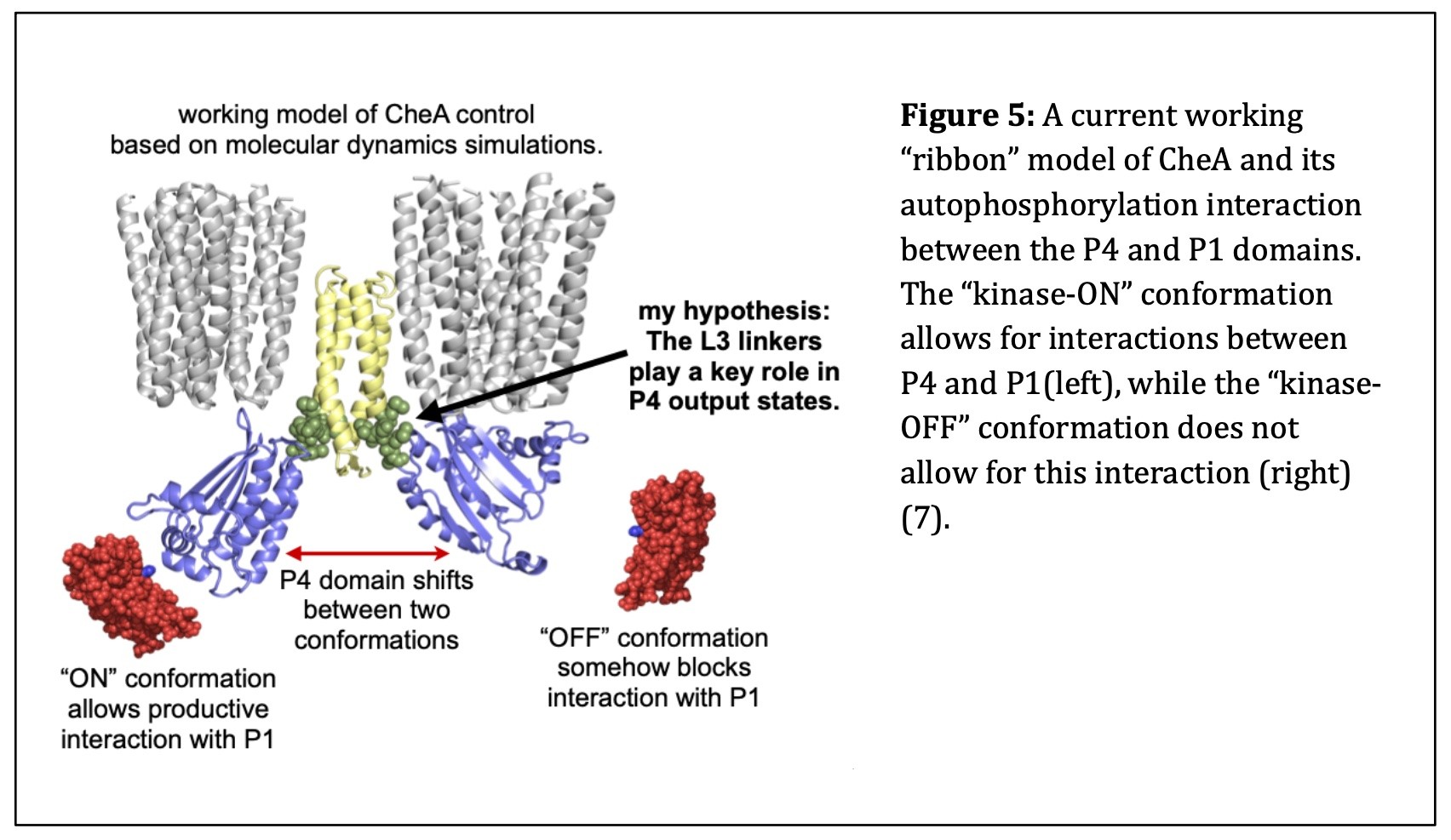
When CheA is in the “kinase-ON” state, its P4 domain must undergo a conformational change that enables it to interact productively with P1, to accept the phosphate group taken from ATP. Once the phosphate group attaches to the P1 domain of CheA, it can be donated to CheY, which then diffuses to the flagellar motors to trigger a clockwise rotational response (8,12). In the “kinase-OFF” state, the P4 domain fails to interact productively with P1. This stops the flux of phosphoryl groups to CheY, inhibiting tumbles and promoting forward swimming. Figure 5 provides a ribbon model of the CheA homodimer in the “kinase-ON” and “kinase-OFF” states based on a working model of CheA control from molecular dynamics simulations.
CheA Linker 3
CheA has an alpha-helical linker, L3, that connects its P3 and P4 domains. L3 is six- residues long and is believed to influence motions of the catalytic P4 domain (5). Whether the L3 linker plays a critical role in the autophosphorylation of CheA is currently under investigation.
Summary
E. coli chemoreceptors on the cell surface transmit stimulus information to a histidine kinase known as CheA (5). CheA has separate functions associated with each of its five domains. Movements of the CheA P4 domain probably control the “kinase-ON” and “kinase-OFF” output states through interactions with the CheA P1 domain (13). L3, a 6-amino acid linker connecting the P3 and P4 domains of CheA, may play a pivotal role in switching P4 between the “kinase- ON” and “kinase-OFF” conformations.
Although other studies have looked at the molecular mechanisms that allow for the various conformations of the P4 domain of CheA, there has not been a study looking specifically at the functional roles of individual L3 residues. My research has the potential to elucidate key structural features of the L3 linker that allow for signaling interactions between the P1 and P4 domains of CheA.
METHODS
Bacterial Strains
I used the E. coli strains UU3269 and UU2784 in my experiments. UU3269 lacks cheA and cheW, but all chemoreceptors are present (Tsr, Tar, Tap, Trg, Aer), cheR/cheB, cheY/cheZ. UU2784 lacks cheA/cheW, cheR/cheB, cheY/cheZ, and Tar, Tap, Trg, and Aer chemoreceptors. Tsr receptors are present in this strain. Below are the complete strain identifications:
UU3269 (cheAW)∆2167 thr(Am)-1 leuB6 his-4 metF(Am)159 rpsL136 [thi-1 ara-14 lacY1 mtl- 1 xyl-5 tonA31
UU2784 (cheA-cheZ)∆1214 (aer)∆5506 ygjG::Gm (trg)∆4543 thr(Am)-1 leuB6 his-4 metF(Am)159 rpsL136 [fliI-I346L(TS) thi-1 ara14 lacY1 mtl-1 xyl-5 tonA31 tsx-78]
{nGEP88 x UU2783/pKD46 >> Rha-r; Kan-s; confirmed by PCR; cured of pKD46; May, 2012} /gp/
Plasmids
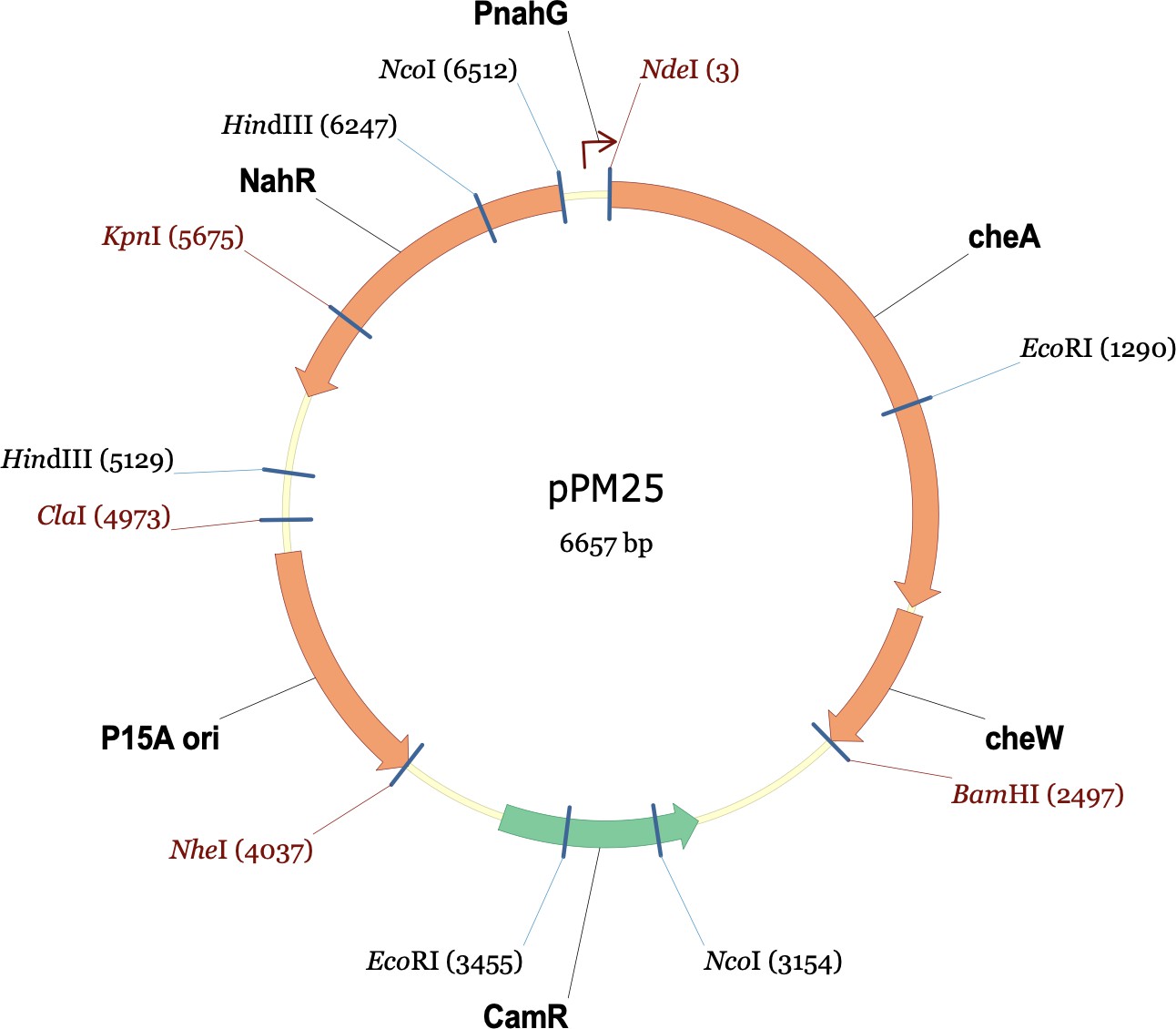
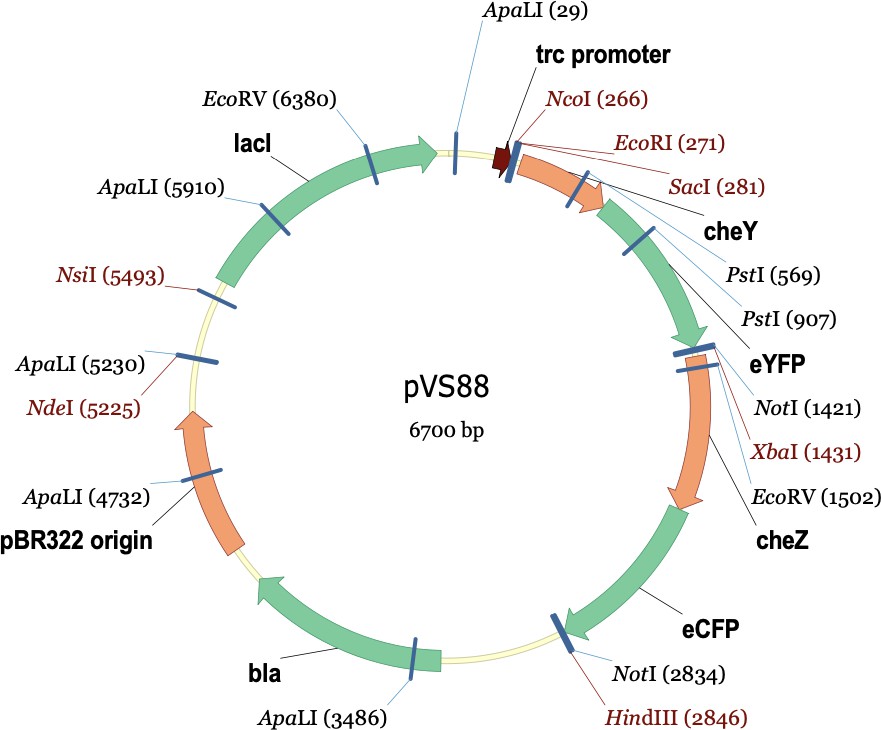
The plasmids used in this experiment were pPM25 and pVS88. pPM25 carries the cheA and cheW genes and confers chloramphenicol resistance. pVS88 carries the cheY and cheZ genes and confers ampicillin resistance. Below are their plasmid maps.
Site-Directed Mutagenesis
I designed forward and reverse primers for each of the five amino acid substitutions I wanted to make at each of the six positions of the L3 linker. I utilized QuikChange PCR reactions to create mutated cheA genes in plasmid pPM25. The mutant plasmid derivatives were transformed into strain UU3269 (∆AW). Ten colonies from each transformation were tested on tryptone soft agar plates (0.3% agar,12.5μg/ml chloramphenicol, 100 μg/ml sodium salicylate) incubated at 37°C to identify the desired mutant candidates.
Protein Expression Assays
Each confirmed mutant plasmid was then subjected to western blotting protocols to determine the cellular expression levels of its mutant CheA protein. Mutant CheA proteins were extracted from the cells and prepared for electrophoresis separation in 10% SDS-PAGE gels.
Each gel was transferred to PVDF membrane paper and exposed to the CheA antibody, followed by treatment with a second antibody that was labeled with the fluorescent dye, Cy5 and able to bind to the CheA antibody molecules in the gel. This allowed for detection of CheA protein by Cy5 fluorescence ImageJ software analysis.
Förster Resonance Energy Transfer (FRET)
Two plasmids, pPM25 (WT or mutant) and pVS88, were transformed into the FRET strain UU2784 (∆AW, ∆YZ). The resulting transformants were then plated on L agar plates (+CAM, +AMP) and incubated at 37°C. Subsequently, overnight cultures were prepared in T- broth (50 μg/ml ampicillin, 25μg/ml chloramphenicol) and left in a shaking incubator at 30°C overnight. Overnight cultures were then subcultured into 10 mL cultures by adding 100 µL overnight culture and 9.9 mL of T-broth media (50 μg/ml ampicillin, 12.5 μg/ml chloramphenicol, 100 μg/ml sodium salicylate, 50 μM IPTG) to a flask. Cells were then washed, centrifuged, and attached to a polylysine coverslip. The coverslip was then attached to a flow cell, kept at 30°C, to allow for various dilutions of serine to flow past the cells. KCN was used as a control to kill all kinase activity. Using Kaliedograph software, Tsr receptor activity was tracked in response to various serine concentrations.
RESULTS
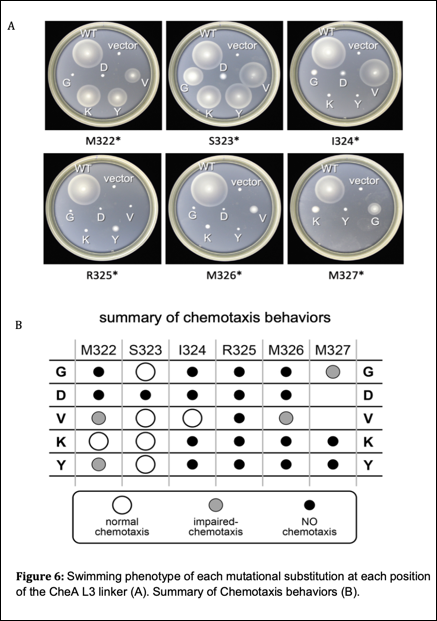
To explore functional properties of CheA linker 3, I created amino acid replacement mutants for each of the six residues in the L3 linker. The amino acid replacements that I chose were glycine, lysine, valine, tyrosine, and aspartic acid. These amino acids were chosen for their various chemical characteristics including size, polarity, charge, and hydrophobicity. I transformed the mutant plasmids into strain UU3269 (∆AW) and assessed their ability to support chemotaxis using soft agar T-swim plates. These plates show the chemotaxis proficiency of each mutant compared to wildtype. Of 28 different L3 mutants, 22 produced defective swimming behavior compared to wildtype (see Figure 6).
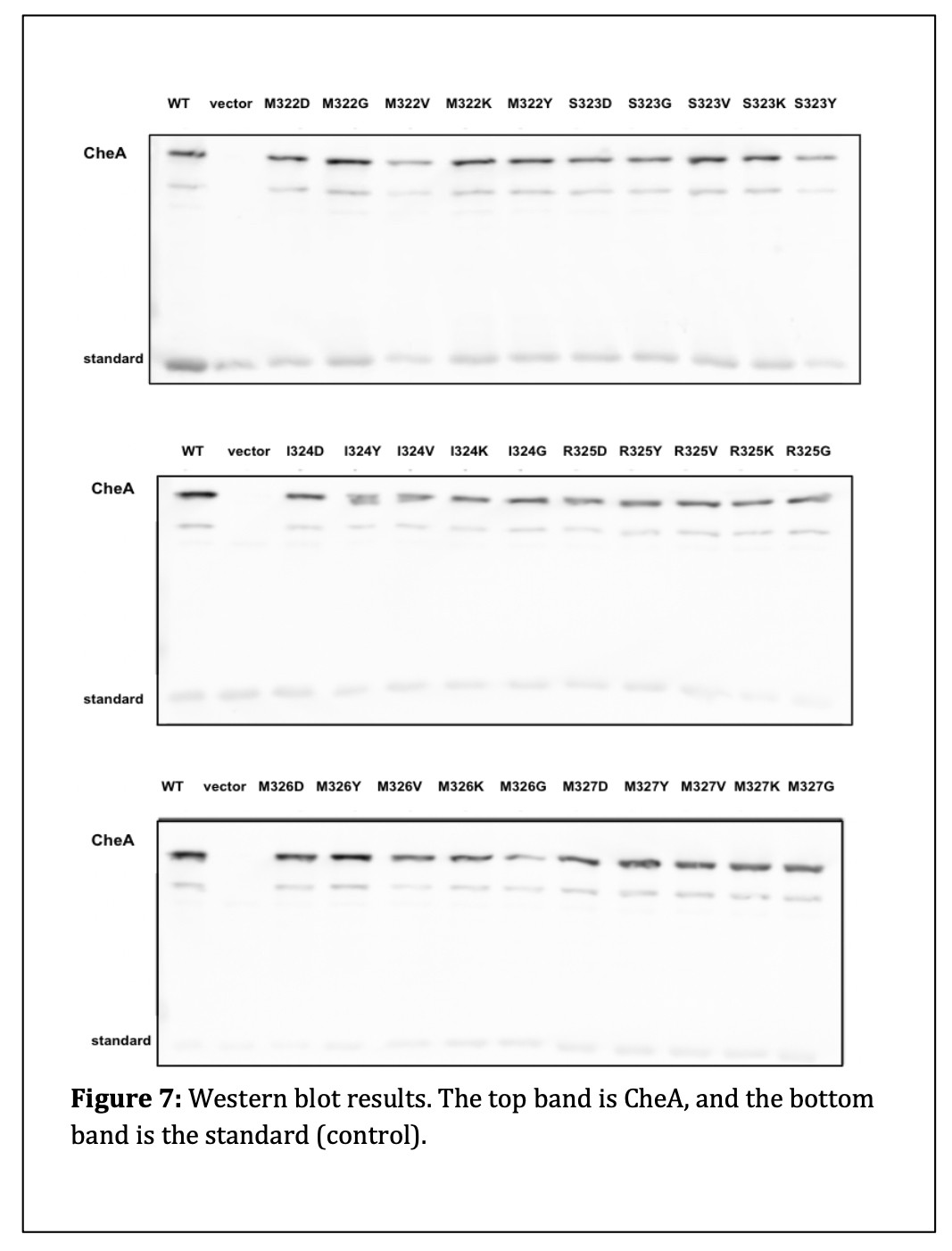
I next measured the cellular amount of protein produced by each L3 mutant. (see Figure 7). I found that there were no significant differences in the levels of protein expression in the L3 mutants compared to wildtype. This result indicates that the L3 amino acid replacements did not alter the expression or stability of their mutant products.
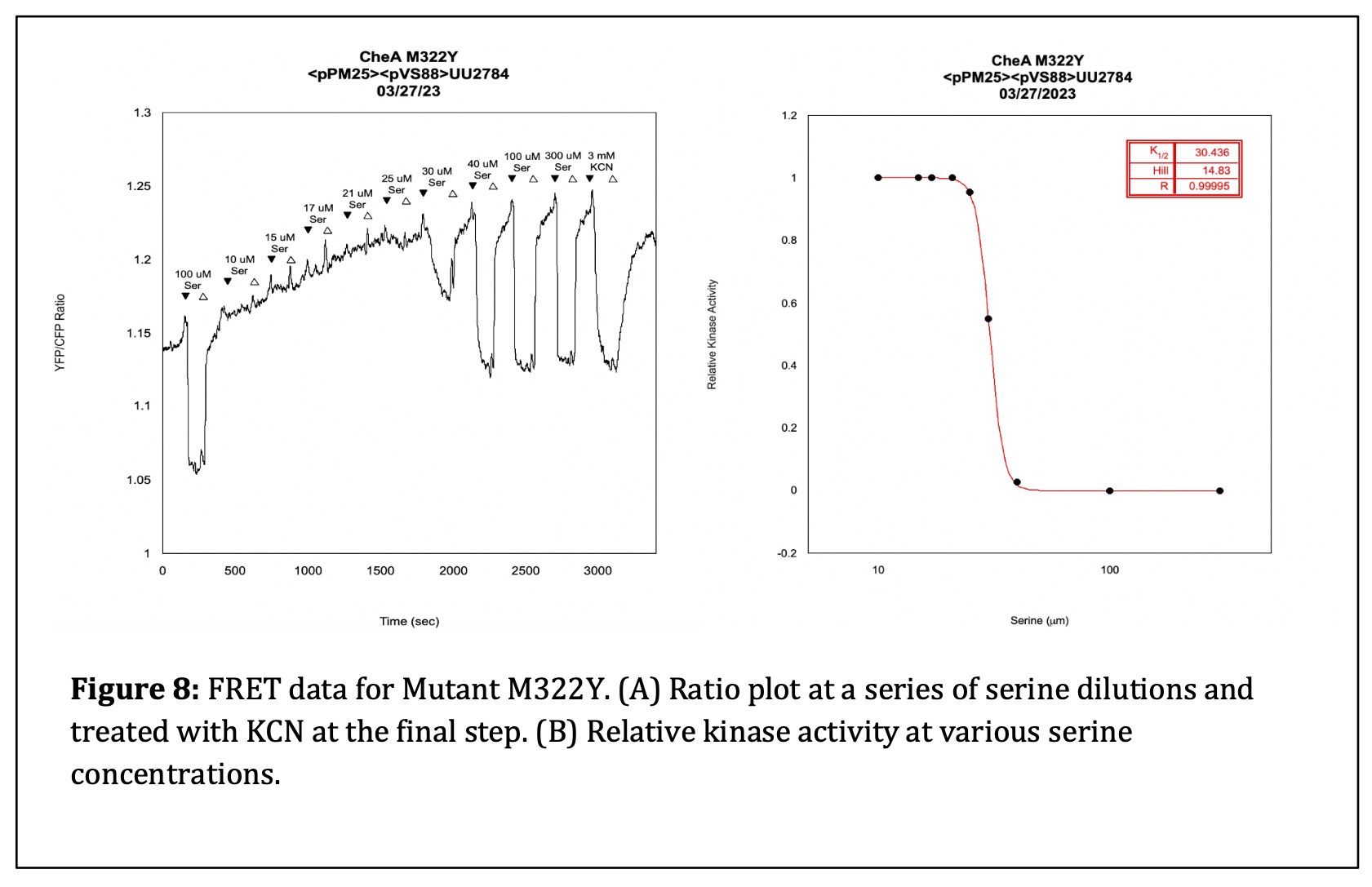
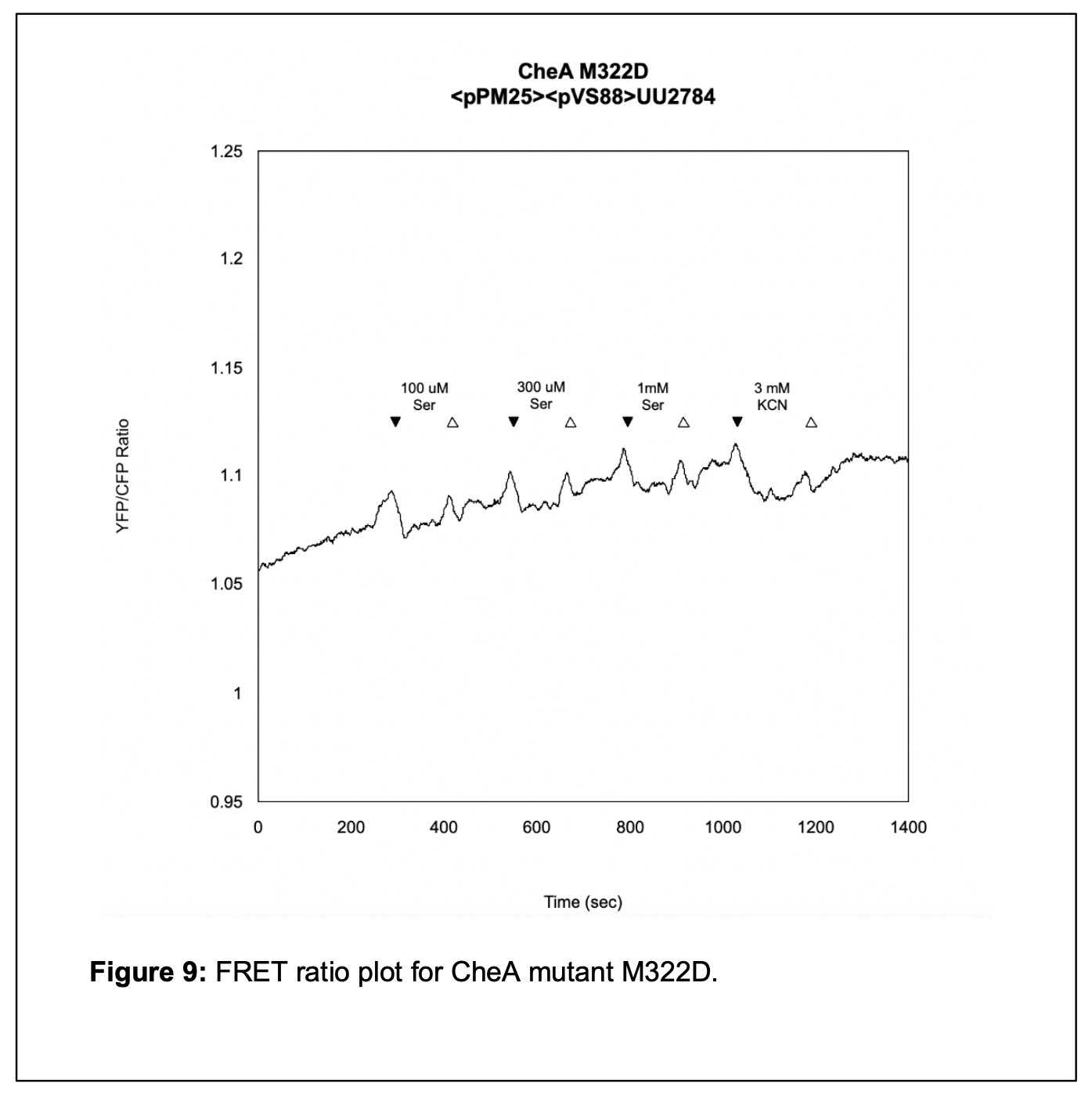
The final step of my initial analysis of the linker mutants was using an in vivo FRET kinase assay to assess Tsr receptor control of the mutant CheA L3 proteins. I transferred my pPM25 mutant plasmids and the FRET reporter plasmid pVS88 into the bacterial strain UU2784. I challenged the cells with various concentrations of serine to determine whether Tsr could activate autophosphorylation of the CheA L3 mutant proteins and, if so, whether that activity could be inhibited by serine stimuli. I found that the M322D mutant could not produce kinase activity. However, the M322Y mutant had kinase activity and responded to serine with a K1/2 value of 30 µM [serine] The K1/2 value specifies the concentration of serine that inhibits ½ of the kinase activity. The K1/2 value of a wildtype strain is approximately 19 µM [serine]. Although the M322Y mutant has nearly wild-type serine sensitivity in the FRET assay, it could not support chemotaxis in the soft agar test. (see Figure 9).
DISCUSSION
To better understand the molecular mechanisms underlying the conformational changes of CheA during its signaling transactions, I created and characterized mutants of CheA-L3, the short, flexible linker connecting the P3 and P4 domain of the CheA protein. I wanted to determine if the L3 linker played an important role in the autophosphorylation interaction between the P4 domain and the P1 domain. This approach enabled me to assess how critical the chemical properties (e.g. acidic, basic, polar, nonpolar, aromatic, charged, small, large, etc.) of the mutant amino acids at each position in the linker were to CheA function (7).
Out of 28 different CheA L3 mutants, 22 had severely impaired swimming behavior. This indicates that L3 function is important for proper chemotaxis and highly sensitive to single amino acid replacements.
Protein expression assays revealed that the protein expression and stability of the mutant proteins were similar to wildtype. Thus, I conclude that CheA L3 mutant phenotypes are not caused by a failure to make the mutant CheA protein.
From the data collected in my FRET experiments, I found that mutant M322Y, with impaired swimming behavior on soft agar T-swim plates, had a K1/2 value of 30 µM [serine] (see Figure 8). This value is only slightly higher than the wildtype K1/2 of 19 µM [serine], which indicates that the M322Y mutant is a bit less sensitive to serine. As seen in Figure 9, mutant M322D, which did not perform chemotaxis on soft agar T-swim plates, did not respond to saturating concentrations of serine and exhibited only a small response to KCN, suggesting it had a kinase-OFF output. This result suggests that CheA L3 plays a significant role in enabling the CheA protein to transition between the “ON” and “OFF” conformational states.
To continue characterization of these CheA mutations and their effect on intracellular signalling, collecting and confirming data from FRET experimentation would be necessary. Expanding my FRET data analysis would help me determine if the nonfunctional CheA mutants lock CheA in the “ON” or “OFF” state. This work could also contribute to the field’s current understanding of amino acid side chain character and structural consequences when protein sequence is altered.
Knowing that L3 is alpha-helical, I am interested in exploring the structural consequences of lengthening the linker by inserting alanine, a residue that holds alpha-helical structures well. This could elucidate the importance of length for proper interaction between the P4 and P1 domains of the CheA protein.
ACKNOWLEDGEMENTS
A special thank you to Dr. Sandy Parkinson and Peter Ames for their help and guidance throughout this research process. Thank you to the ACCESS Program for Women in Science and Mathematics and a grant from the National Institute of Health to the Parkinson Lab for funding my work.
REFERENCES
- S. Bi, V. Sourjik, Stimulus sensing and signal processing in bacterial chemotaxis. Curr.Opin. Microbiol. 45, 22-29 (2018).
- G. L. Hazelbauer, Bacterial chemotaxis: the early years of molecular studies. Annu Rev Microbiol 66, 285-303 (2012).
- C. K. Cassidy et al., Structure and dynamics of the E. coli chemotaxis core signaling complex by cryo-electron tomography and molecular simulations. Commun Biol 3, 24 (2020).
- C. H. Hansen, R. G. Endres, N. S. Wingreen, Chemotaxis in Escherichia coli: a molecular model for robust precise adaptation. PLoS Comput Biol 4, e1 (2008).
- A. R. Muok, A. Briegel, B. R. Crane, Regulation of the chemotaxis histidine kinase CheA: A structural perspective. Biochim Biophys Acta Biomembr 1862, 183030 (2020).
- R. B. Sekar, A. Periasamy, Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol 160, 629-633 (2003).
- J.S. Parkinson, Bacterial Chemotaxis, in Brenner’s Encyclopedia of Genetics (Second Edition), Academic Press, pp. 258–261(2013)
- . A. M. Bilwes, C. M. Quezada, L. R. Croal, B. R. Crane, M. I. Simon, Nucleotide binding by the histidine kinase CheA. Nat. Struct. Biol. 8, 353-360. (2001).
- H. Huo, R. He, R. Zhang, J. Yuan, Swimming Escherichia coli cells explore the environment by Levy walk. Appl Environ Microbiol 87 (2021).
- Z. D. Blount, The unexhausted potential of E. coli. Elife 4 (2015).
- M. Torres, H.J. Forman, “Signal Transduction” in Encyclopedia of Respiratory Medicine pp. 10-18 (2006).
- K. Jahreis, T. B. Morrison, A. Garzon, J. S. Parkinson, Chemotactic signaling by an Escherichia coli CheA mutant that lacks the binding domain for phosphoacceptor partners. J. Bacteriol. 186, 2664-2672 (2004).
- A. Garzón, J. S. Parkinson, Chemotactic signaling by the P1 phosphorylation domain liberated from the CheA histidine kinase of Escherichia coli. J. Bacteriol. 178, 6752- 6758 (1996).
- J. S. Parkinson, G. L. Hazelbauer, J. J. Falke, Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol 23, 257-266 (2015).
- C. E. Flack, J. S. Parkinson, Structural signatures of Escherichia coli chemoreceptor signaling states revealed by cellular crosslinking. Proc Natl Acad Sci USA 119, e2204161119 (2022).
- X. Chen, S. Bi, X. Ma, V. Sourjik, L. Lai, Discovery of a new chemoeffector for Escherichia coli chemoreceptor Tsr and identification of a molecular mechanism of repellent sensing. ACS Bio Med Chem Au 2, 386-394 (2022).
Media Attributions
- thumbnail_image009
- thumbnail_image008
- thumbnail_image007
- thumbnail_image006
- thumbnail_image005
- thumbnail_image004
- thumbnail_image003
- thumbnail_image002
- thumbnail_image001

