College of Pharmacy
86 Effect of Great Salt Lake Dust on Transient Receptor Ion Channels and Lung Epithelial Cells
Caitlin Pham and Christopher Reilly
Faculty Mentor: Christopher Reilly (Pharmacology and Toxicology, University of Utah)
Abstract
As the Wasatch Front experiences more frequent droughts, a growing local concern is the resuspension of Great Salt Lake Dust (GSLD) into the surrounding air from the exposed lakebed. It is suggested that GSLD represents an emerging environmental health threat, yet its effects and the underlying mechanisms driving adverse health impacts remain poorly defined. This study aimed to characterize the inflammatory and cytotoxic potential of GSLD using human bronchial epithelial cells as a model for inhalation exposure to GSLD, and the role of transient receptor potential (TRP) channels in mediating particle-induced inflammatory responses and cytotoxicity. The role of elemental composition and the modulatory effects of adenosine, a chemical constituent of GSLD, were also evaluated. BEAS-2B cells were treated with GSLD and dust from the Salton Sea, another saline lake, to evaluate whether dust-induced cellular responses were consistent across geographically distinct but compositionally similar sources. After assessing effects on cell viability, calcium signaling, and cytokine expression, results showed that GSLD induced stronger cytotoxicity and calcium flux than SSD, consistent with TRP channel activation. While both dusts induced pro-inflammatory cytokine expression as measured through IL-6 and IL-8 mRNA expression, a TRPV3 antagonist 007 only inhibited IL-6, while a TRPV1 antagonist LJO-328 inhibited both IL-6 and IL-8, suggesting channel-specific contributions to cytokine regulation. Adenosine, investigated for the first time as a possible TRPV1 antagonist and natural component of GSLD, significantly attenuated cytokine expression at higher concentrations, supporting prior suggestions that it may be a TRPV1 antagonist and that compounds associated with GSLD and similar dusts, including adenosine, may modulate TRP-mediated inflammatory responses to environmental dusts. Differences in dust source location and particle size did not significantly impact inflammatory outcomes, and compositional analysis using ICP-MS to determine elemental composition did not show evidence of a cause-effect relationship for any specific element tested. These findings collectively underscore the importance of specific chemical and biological components—such as adenosine—and interactions with specific cellular receptors in shaping cellular responses to dusts. Taken together, this study highlights the complex interplay between particulate matter composition and TRP channel-mediated stress responses, informing future research aimed at mitigating dust-induced inflammation and/or injury of airway epithelial cells and respiratory conditions impacted by inhaled dusts such as GSLD and SSD.
Introduction
Great Salt Lake Dust and Salton Sea Dust
Air pollutants are known to cause lung inflammation, tissue damage, and the onset and exacerbation of asthma. A growing concern is Great Salt Lake Dust (GSLD), which is resuspended from lakebed sediment that contains a variety of salts, natural and anthropogenic pollutants, biological components, and even human pathogens. As a terminal lake, the Great Salt Lake is prone to accumulating pollutants because there is no outlet. When the Great Salt Lake loses water, larger areas of lakebed become dry and amenable to resuspension during high wind events, leading to pollutants such as arsenic, cadmium, mercury, nickel, chromium, lead, copper, selenium, organic contaminants, and cyanotoxins being transported through the air (Abbott et al., 2023). Inhaling this dust can put people at risk of developing acute and chronic respiratory illnesses. Supplemental Figure 1 illustrates such a dust event on April 21, 2022.
Residents of the Salt Lake Valley are also routinely exposed to many forms of environmental air pollutants, such as Coal Fly Ash (CFA), geological dusts, wood/wildfire smoke, industrial emissions, and diesel/vehicle exhaust. These particulate materials (PM) cause adverse effects on human health. PM2.5 is of concern because it often exceeds the EPA standard for “unhealthy” air quality in the Salt Lake Valley during the winter (Lamb et al., 2017). High concentrations of PM2.5 can trigger respiratory emergencies in already sensitive populations, such as the elderly, children, and asthmatics. PM2.5-10 has also been linked to increased risks for illnesses such as asthma, COPD, lung cancer, and other cardiovascular diseases. The composition of PM2.5 varies by location, aging, and origin, which can lead to different health effects through unique mechanisms. This highlights the need to study how various pollutant sources interact with and trigger adverse responses in target tissues. Dust events are linked to an increase in emergency room admissions, with data showing the number of ER visits per 10,000 people in each region following a dust event, along with the GSLD collection sites, emphasizing the public health impact of these pollutants (Supplemental Figure 2).
The Salton Sea is a similarly shrinking saline lake in California that also has recorded dust events. Salton Sea sediments are known to contain pesticides and toxic metals such as selenium, cadmium, molybdenum, nickel, and zinc, and the significant decline in water level that is predicted between 2018 and 2030 will only exacerbate these effects (Frie et al., 2017). Like the Great Salt Lake, the Salton Sea is a terminal body of water without outflow, which allows for the accumulation of metals and pollutants. It has been shown that the exposed lakebed can release toxic chemicals such as hydrogen sulfide, arsenic, and pesticides into the air, affecting the air quality of the neighboring metropolitan areas of Los Angeles and San Diego (Marshall 2017). Since GSLD and SSD both originate from desiccating saline lakes and share similar particle characteristics, their elemental compositions are relatively similar, with only small differences (Supplemental Figure 3). Comparing their effects provides further insight into the broader health impacts of environmental dust exposure across geographically distinct regions.
A

B

Figure 1A. Photograph of Great Salt Lake dust impacting the west side of the Salt Lake valley. The photo was taken from Skaggs Pharmacy Research Building at the University of Utah by Dr. Christopher Reilly. Figure 1B. Photograph of a dust storm at the Salton Sea, California, obtained from UC Riverside News.
TRP Ion Channels
Activation of transient receptor potential (TRP) ion channels, or TRP channels, by inhaled air pollution particles causes lung inflammation, among other effects. TRP channels are found in almost all cells throughout the body and can be activated by stimuli such as temperature, pain, and certain chemicals. TRPV1 (vanilloid-1) and TRPV3 (vanilloid-3) are activated by capsaicin and carvacrol, and play roles in thermal sensation and pain, among other processes (Zhang et al., 2023). TRPV1 activation in the lungs causes coughing, while another channel, TRPV3, regulates human bronchial epithelial cell proliferation and wound repair (Nguyen et al., 2020). TRPA1 (ankyrin-1), another TRP channel of interest, is the only TRPA protein found in humans, and it is relevant to a wide array of diseases due to its sensitivity to many types of external stimuli, such as cold temperatures and chemical irritants (Zhang et al., 2023). Because of their important roles in sensory biology and inflammation, TRP ion channels are being explored as targets for drug development.
TRP channels in general serve as molecular detectors of harmful environmental stimuli. Prior studies have shown that exposure to airborne capsaicinoids—like those found in pepper spray—induce acute respiratory inflammation and epithelial damage through TRPV1 activation (Reilly et al., 2003). Immortalized human airway epithelial cells, such as BEAS-2B and A549 cells, express TRPV1 mRNA. Higher transcript levels correlate with increased sensitivity to capsaicinoids, measured using pro-inflammatory cytokine mRNA induction and cytotoxicity, suggesting that TRPV1 expression levels may influence cellular vulnerability to particle/PM2.5-induced damage. TRPV1 overexpression in bronchial epithelial cells significantly amplified inflammatory response and apoptotic cell death, further confirming its role in mediating these responses. These findings support the relevance of TRP channels as environmental sensors that transduce harmful signals into inflammatory and toxicological outcomes in the respiratory tract.
Calcium Signaling and Endoplasmic Reticulum Stress
A key mechanism of TRPV1 activation is the influx of calcium ions (Ca²⁺), which disrupts intracellular calcium homeostasis and can trigger downstream stress responses (Deering-Rice et al., 2018). Exposure to capsaicinoids caused calcium-dependent production of IL-6, a proinflammatory cytokine, while calcium chelation using EGTA ablated this effect, linking calcium flux directly to cytokine expression (Reilly et al., 2003). Cytotoxicity could also occur independently of extracellular calcium, via endoplasmic reticulum calcium depletion, endoplasmic reticulum stress (ERS) and cell death (Reilly et al., 2003). These results highlight how TRPV1-mediated calcium flux can initiate inflammatory signaling and cell damage, making it a key area of interest for understanding dust-induced respiratory toxicity.
Another study conducted by Nguyen et al., 2020 suggested that particulate matter (PM) can activate multiple TRP channels, triggering downstream cellular responses such as cytokine release, ERS, and cytotoxicity. Exposure to pine wood smoke particulate matter (WSPM) resulted in decreased TRPA1 expression and increased TRPV3 expression, changes that were associated with reduced ERS and cell death. These findings suggest that TRPA1 may contribute to ERS under certain PM exposures, while TRPV3 activation may play a protective role by dampening these stress-related pathways.
Several TRP channels function as calcium-permeable conduits, and their activation is closely tied to inflammatory and stress responses. For instance, in human corneal epithelial cells, TRPV1 activation triggers calcium influx, which initiates MAPK signaling and results in elevated IL-6 and IL-8 release key cytokines also associated with ERS (Zhang et al., 2023). IL-8 is a chemokine that attracts neutrophils to sites of irritation or damage, and along with IL-6, is associated with ERS and cytotoxicity, making them useful indicators of cell stress in response to particulate matter (Tanaka et al., 2014). These findings suggest that TRP channel activation can play a key role in promoting inflammation and stress responses in epithelial cells.
Research Objectives
This project tested the hypothesis that GSLD and similar lakebed dusts would interact with TRP channels such as TRPV1 to promote inflammation and/or cell death using BEAS-2B human airway epithelial cells a model cell target of inhaled dusts. To investigate this, experiments measured dose-dependent cytotoxicity of GSLD and SSD using a viability assay and assessed TRP-channel dependent calcium flux on cells treated with GSLD and SSD compared to other known positive controls for TRP channels.
Puntambekar et al. (2014) found that analogues of adenosine could inhibit TRPV1- mediated calcium flux in HEK-293 cells stably expressing TRPV1, suggesting that adenosine may also inhibit TRPV1 in airway epithelial cells or other relevant pulmonary models. As a naturally occurring component of GSLD (Supplemental Figure 4), adenosine was thus tested as a potential antagonist for the first time in our lab, alongside other known antagonists of TRPV1 and TRPV3, to assess its potential to inhibit pro- inflammatory cytokine expression.
The study also aimed to examine the impact of GSLD particle size and geographic origin on cytokine expression in airway epithelial cells. To support this investigation, GSLD samples underwent ICP-MS analysis to determine whether compositional differences in trace metal content between samples of different sizes and locations may drive differential inflammatory responses. Collectively, this work contributes to understanding how complex environmental particulates like GSLD interact with airway epithelial cells to promote inflammation and cell death.
Methods
Chemicals
The TRPA1 and TRPV3 agonist carvacrol was purchased from Sigma-Aldrich. The TRPV1 agonist nonivamide, a capsaicin analogue, was purchased from Cayman Chemical. Dr. Jeewoo Lee from Seoul National University provided the TRPV1 antagonist LJO-328. The TRPV3 antagonist 2-(5-trifluoromethyl-pyridine-2ylsulfanyl)-1-(8-methyl-3,4- dihydro-2H-quinolin-1-yl)-ethanone (abbreviated as 007) was synthesized as described by Deering-Rice et al., 2014. Adenosine was also tested as a potential TRPV1 antagonist (Puntambekar et al., 2004). Adenosine was purchased from Sigma Aldritch and diluted to 50 mM in DMSO to prepare a stock solution to be used for all experiments.
Cell Culture and Treatment
BEAS-2B cells are an SV40 T-antigen immortalized bronchial epithelial cell line that models selected aspects of human airway epithelial cell responses to air pollution particles. For experiments, cells were plated in LHC9 media (Lechner and LaVeck medium; Life Technologies) for 48 hours prior to treatments (Lamb et al., 2017). The cells were then treated with GSLD and SSD suspended in LHC9 cell culture media for 24 hours. Supplemental Figure 5 shows microscope images comparing different GSLD samples at the timepoint of initial treatment vs 24 hours. LHC9 was the negative control for most experiments, while others included a low concentration of DMSO. Cell treatments containing GSLD or SSD also included 1X Pen-Strep, an antibiotic containing penicillin G and streptomycin that prevented bacterial contamination from the non-sterile dusts. For experiments using GSLD/SSD co-treated with an antagonist, cells were treated first with the antagonists for 1 hour, then with a combination of the antagonists and dusts for 24 hours, at which point the treatments were removed and the cells frozen for future analysis.
Dust Collection
The Reilly Lab collected dust samples from 7 locations along the Great Salt Lake shoreline, UT, and 2 locations at the Salton Sea, CA. From these locations, and from pools of materials, GSLD and SSD were size-fractionated using a 10-stage Andersen cascade impactor as described by Cowley et al. (2025). Stages 4-7 were combined to be used as PM2.5 for cell treatments in all cell-based experiments, except for experiments where size and location were tested as the independent variables (Figures 6 and 7).
For the experiment testing particle size, a mixture of GSLD was size fractionated through the cascade impactor, with particle sizes depicted and quantified in Supplemental Figure 6. Each of the 7 stages, plus the pre-separatory stage and final stage, were kept isolated in separate collection bottles. For the experiment testing different site locations, each site location was size fractionated and stages 4-7 were combined for each location.
Dojindo Viability Assay
For the viability assay, cells were plated at 20k/cm2 in a 96 well plate for 48 hours, then treated with dust concentrations ranging from 0-15 mg/ml in a 12-step serial 2x dilution for 24 hours. Viability was determined using the Dojindo reagent, a dye that turns orange when metabolized by live cells (Veranth et al., 2007). After a 30-minute treatment, a gradient in color was observed indicating that higher concentrations of dust caused cells to die and appear (i.e., the reagent solution was less orange). The absorption of the reagent was measured at 450 nm using a Molecular Devices plate reader. Data was normalized by dividing each value by the mean of the vehicle control group and expressed as fold change relative to control.
Calcium Flux Assay
Calcium flux assays are used to quantify TRP channel activation by measuring the changes in intracellular calcium (i.e., fluorescence intensity) in response to various treatments. To measure this, the BEAS-2B cells were incubated for 1 hour in Fluo-4-AM loading solution, a cell permeable fluorescent dye that is de-esterified in cells to yield a chelator that fluoresces when bound to calcium. Cells are then washed with a calcium buffer containing 0.75 mM Trypan Red and 1 mM probenecid to facilitate dye retention and to quench background fluorescence before imaging. An EVOS FL Auto Imaging System (Life Technologies) was used to measure changes in fluorescence immediately following treatment. The TRP agonists carvacrol and nonivamide were used as positive controls at concentrations of 500 μM. GSLD and SSD treatments were at concentrations of 180 μg/cm. The data were normalized to the percent of maximum possible fluorescence induced by 10 μM ionomycin, a calcium ionophore that induces the maximum calcium influx.
qPCR Gene Expression Analysis
For all qPCR experiments, cells were plated at 25k/cm2 for 48 hours in 1 mL/well LHC9 media. They were then treated for 24 hours in 1 mg/ml of dust. Each treatment condition had 3 biological replicates. For experiments with co-treated cells, the cells were pre-treated with the antagonists for 1 hour. Subsequent co-treatments with the antagonist and dust were added to incubate for 24 hours. Antagonists included 20 μM LJO-328 and 50 μM 007, the same concentrations used by Cowley et al. on the HBEC3-KT cells. Adenosine was used at concentrations of 50 μM, 0.5 mM, and 1 mM. Dust co-treatments were at concentrations of 1 mg/ml of GSLD or SSD. Following the 24 hour treatment, the media was aspirated off and the cells frozen at –80 °C until ready for RNA extraction.
Total RNA was isolated from the cells using PureLink RNA Mini Kit (Invitrogen; Carlsbad, CA) and stored at –80 °C. The RNA was then reverse transcribed into complementary DNA using the ABI High-Capacity cDNA Kit + RNase Inhibitor (Applied Biosystems, Foster City, CA) and stored at -4 °C.
Gene expression of IL-6 and IL-8 was measured using two-step reverse transcriptase quantitative PCR (qPCR), via amplification of cDNA using the QuantStudio Real-Time PCR System with TaqMan™ Gene Expression Master Mix and gene-specific TaqMan probes. Gene expression was normalized to B2M, (β2-microglobulin) a housekeeping gene that is not affected by experimental treatments, environmental conditions, or disease states. IL-6 and IL-8 expression levels were analyzed using the ΔΔCt method to assess relative changes across treatment groups.
ICP-MS
20 mg samples were prepared for each of the 10 size fractions of GSLD, as well as the 7 different site locations for GSLD and 2 sites for SSD. These samples were then sent to the University of Utah Iron and Heme Core Facility for ICP-MS analysis using an Agilent 7900 spectrometer operated in He collision mode as described by Cowley et al. (2025) to determine potential differences in elemental composition between the different size fractions and different site locations that may inform of mechanisms that impact potency as pro-inflammatory and/or cytotoxic agents.
Statistical Analysis
Data is represented as mean of biological replicates ± SD. Significance was determined using a one-way ANOVA with Bonferroni post hoc correction at the 95% confidence interval, unless otherwise stated.
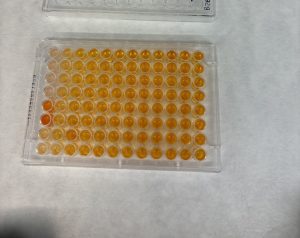
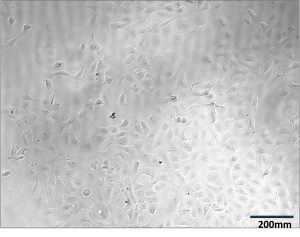
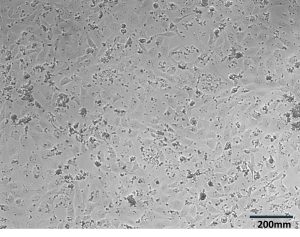

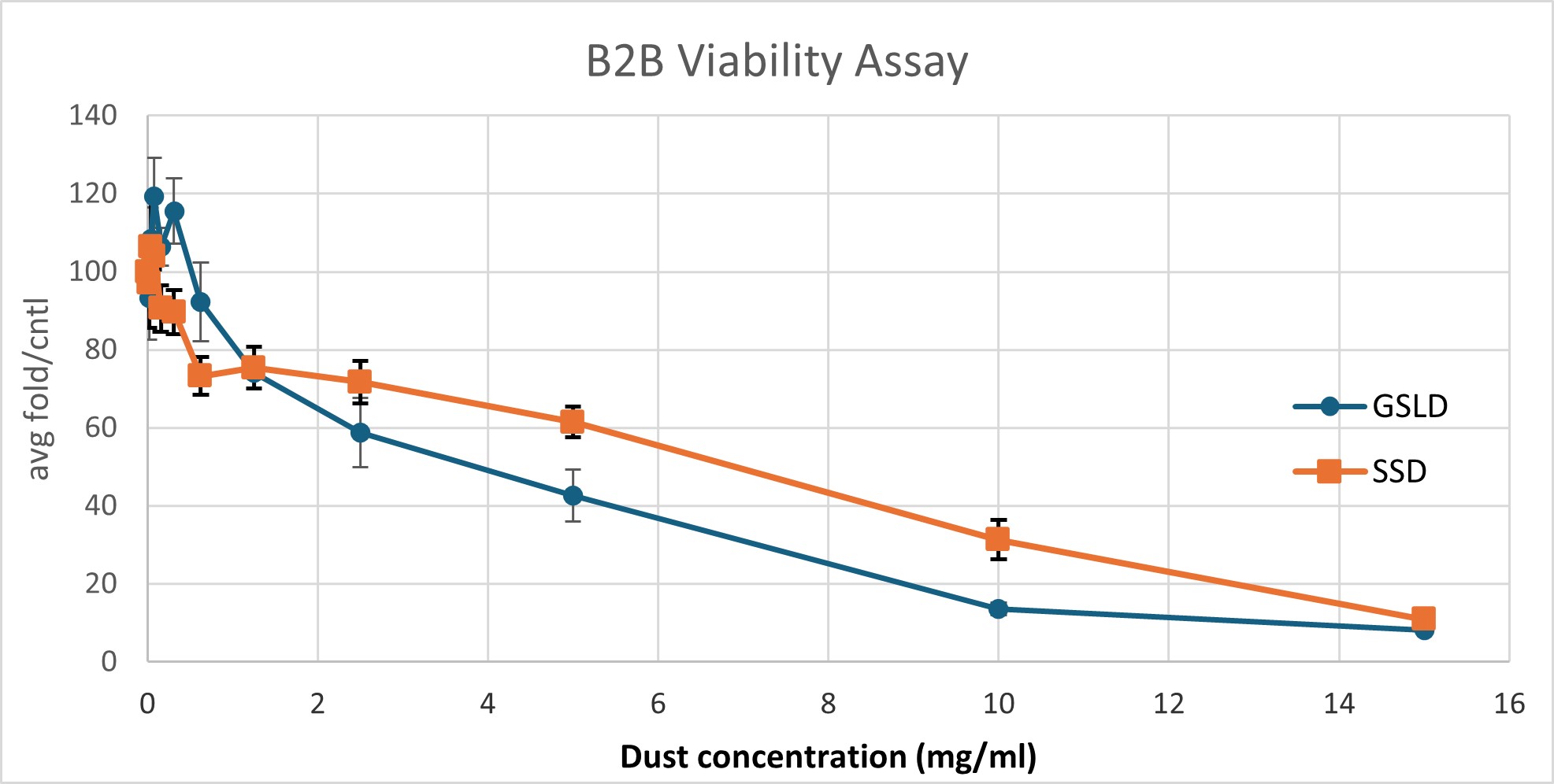
Figure 2. GSLD and SSD cytotoxicity using a Dojindo assay. Figure 2A. The top four rows contained GSLD, and the bottom 4 contained SSD, with dust concentration starting at 15 mg/ml on the left, then 10 mg/ml, then decreasing by half until the lowest concentration at 0.01935 mg/ml. The lighter color wells indicate that the treatment was acutely cytotoxic. Figure 2B. Untreated BEAS-2B cells under a microscope after 24 hours. Figure 2C. BEAS-2B treated with 0.3125 mg/ml GSLD. Cells are still visible beneath the dust. Figure 2D. BEAS-2B treated with 10 mg/ml SSD. Cells are completely obscured from view. All images were taken using a 10× objective, and a scale bar is included for reference. Figure 2E. Results from repeated trial of the Dojindo, but with the addition of PenStrep. Statistical significance at 2.5 mg/mL was assessed using a one-tailed unpaired t-test of 4 replicates; GSLD was more cytotoxic than SSD (p = 0.02).
GSLD and SSD were cytotoxic to BEAS-2B cells. Previously, it was not known at which dust concentrations that BEAS-2B cells should be treated with to induce a cytotoxic response or the relative potency of these dusts as cytotoxic agents. Serial dilution demonstrated that BEAS-2B cells responded in a similar manner to the HBEC3-KT cells used in previous studies as a lung cell model (Cowley et al., 2025), with 60-70% viability at around 1 mg/ml, the concentration used for all subsequent experiments.
In Figure 2A, the dark orange wells for SSD at the highest concentration suggest that bacterial contamination was present in the dust samples, causing the bacteria to metabolize the Dojindo agent which resulted in the darker colored wells despite higher concentrations being the most cytotoxic. This necessitated a repeat experiment with the addition of an antibiotic cocktail, PenStrep, to ensure that bacteria contamination did not affect experimental results (Figure 2E). PenStrep was also added to the cell media in all future dust treatments.
The results from Figure 2E demonstrated that cell viability decreased exponentially as the dust concentration increased, reaching about 10% of cells alive at 15 mg/ml. At concentrations of 1-15 mg/ml, SSD was slightly more toxic to the BEAS-2B cells compared to GSLD, but at concentrations of less than 1 mg/ml, GSLD was more toxic. At 2.5 mg/mL, the approximate LD50 of GSLD, GSLD was significantly more cytotoxic than SSD. Microscope images (Figure 2B–D) support these findings by showing that higher concentrations of dust, particularly SSD, appeared to completely suffocate cells, contributing to cytotoxicity.
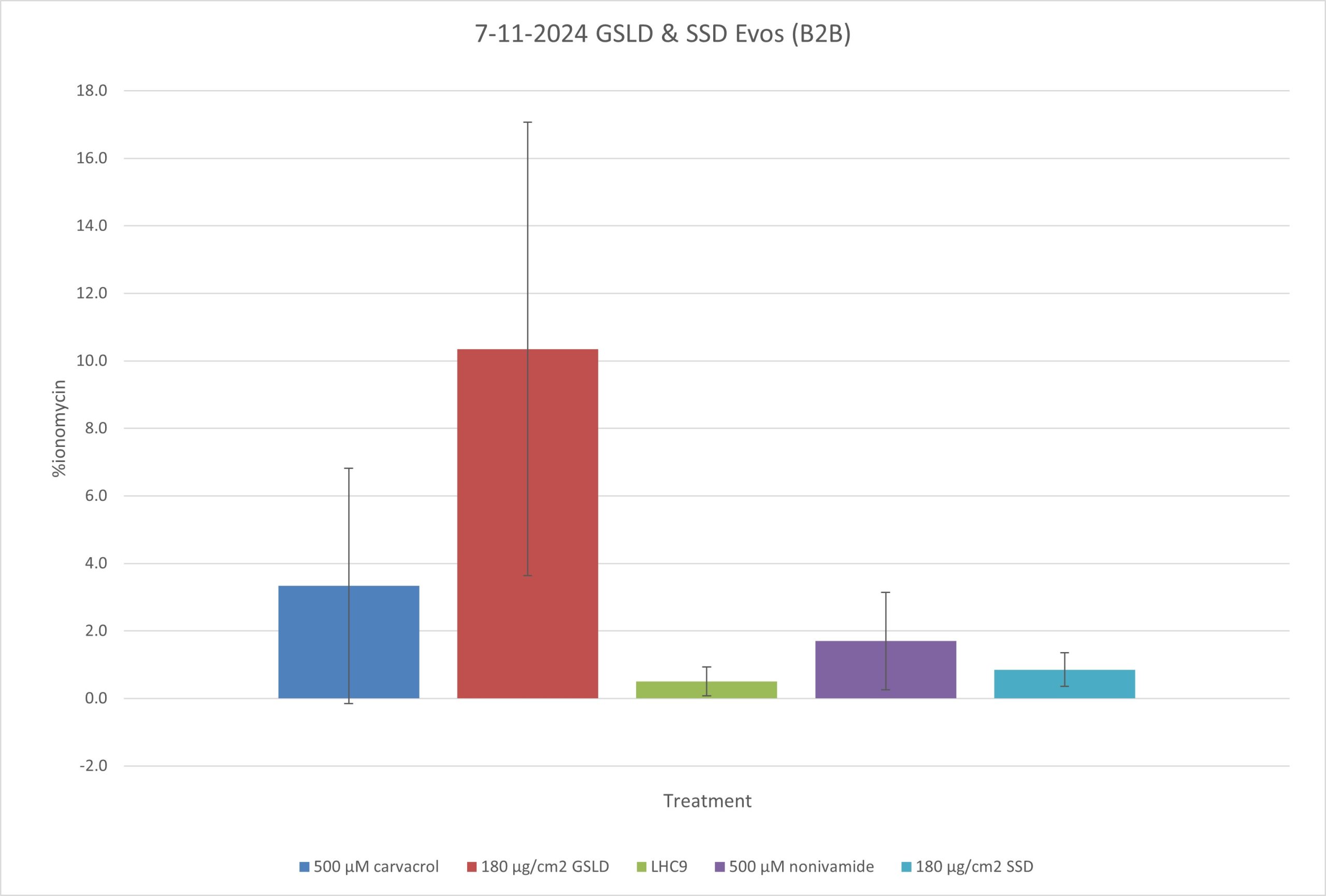
Figure 3. Calcium flux assay with BEAS-2B cells treated with agonists. The graph represents the mean ± SD of 3 biological replicates. GSLD and SSD were compared to the positive controls carvacrol and nonivamide, which are known to induce calcium flux through their respective ion channels (TRPA1 and TRPV1). LHC9 media was the negative control. Relative calcium influx was normalized to ionomycin as previously described in Methods. Statistical comparisons were relative to the control group LHC9 using one-way ANOVA with a Bonferroni correction. *p < 0.05, **p < 0.01.
GSLD induced calcium flux in BEAS-2B cells. GSLD treated cells demonstrated the greatest levels of calcium flux at a statistically significant difference. Comparatively, SSD did not appear to induce calcium flux when compared to LHC9. The positive controls of carvacrol (TRPA1 and V3) and nonivamide (TRPV1) appeared to induce calcium flux, though the difference was not statistically significant. Altogether, this supports the hypothesis that activation of TRPV1, V3, and/or A1 may be important in mediating responses of the cells to GSLD and SSD.
A
B
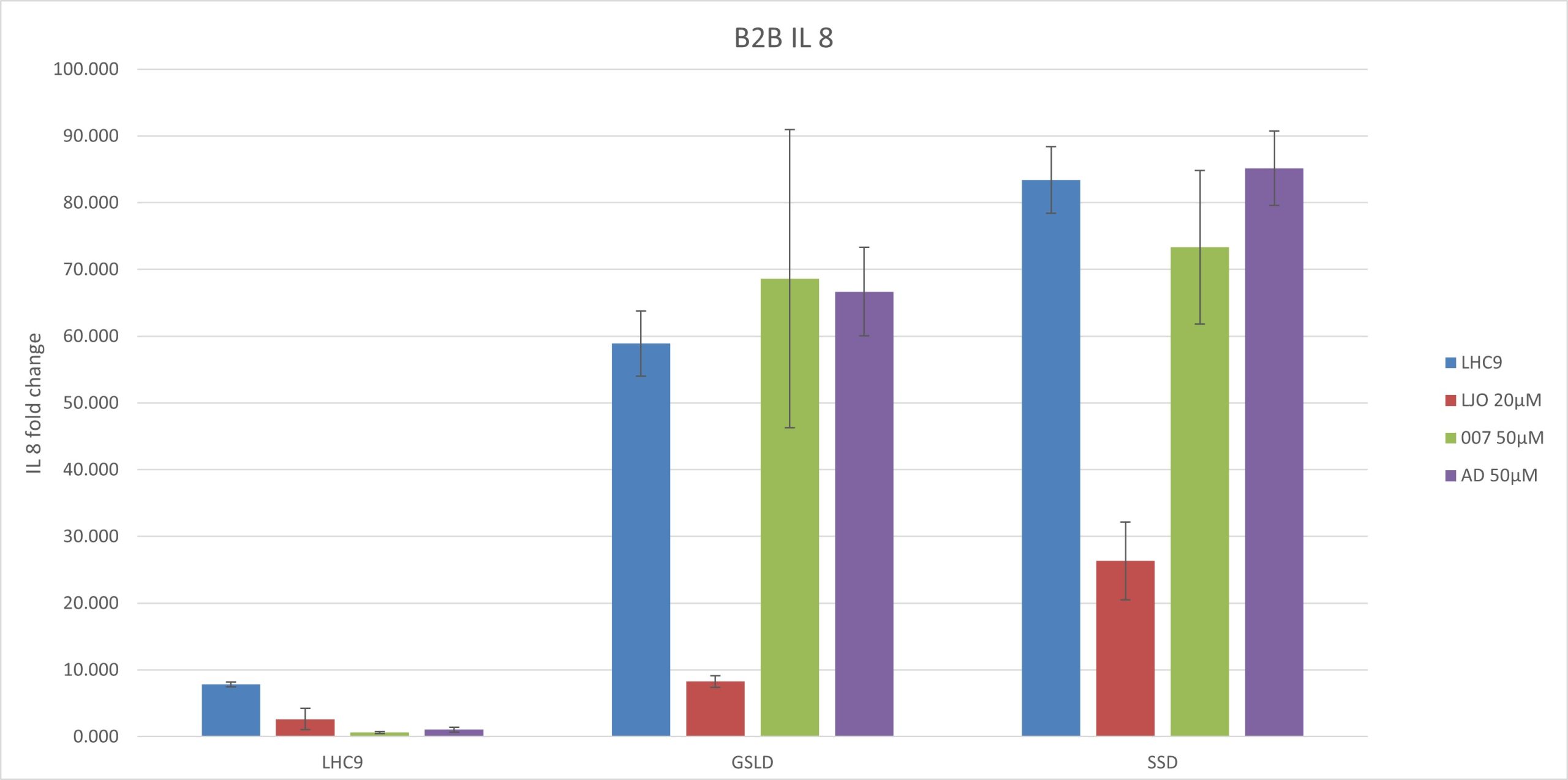
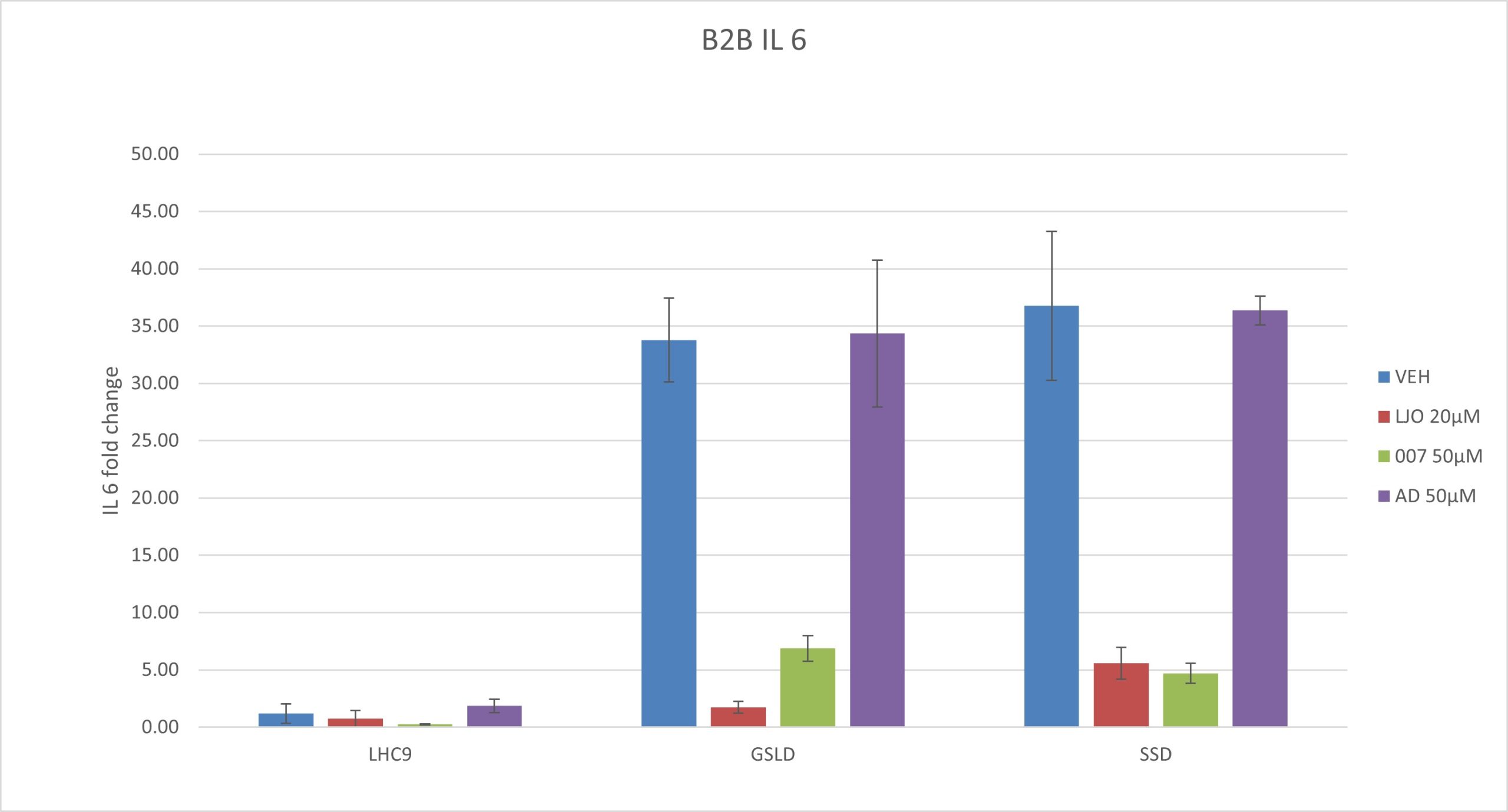
Figure 4. Results of a qPCR experiment conducted on BEAS-2B cells cotreated with GSLD/SSD as agonists and adenosine (TRPV1), LJO-328 (TRPV1), and 007 (TRPV3) as antagonists. Adenosine (AD) was tested at a starting concentration of 50 μM, the same concentration used by Puntambekar et al. (2014). Statistical comparisons were made to the respective vehicle controls for GSLD and SSD, while LHC9 controls were compared to GSLD + media for significance. ***p < 0.001,****p < 0.0001. (A) IL-6 gene expression normalized to B2M. (B) IL-8 gene expression normalized to B2M.
GSLD and SSD induced IL-6 and IL-8 expression via TRPV1 and TRPV3. SSD had a slightly stronger response than GSLD with respect to the induction of mRNA for both IL-6 and IL-8. LJO-328 successfully inhibited both IL-6 and IL-8 induction caused by GSLD and SSD in. With IL-8, 007 failed to inhibit both GSLD and SSD, whereas it did inhibit IL-6. This suggests that 007 selectively inhibits IL-6 but does not affect IL-8 expression induced by either GSLD or SSD, indicating a potential difference in the mechanisms regulating IL-6 and IL-8 induction.
Adenosine (50 μM) did not inhibit cytokine response. Adenosine failed to inhibit IL-6 or IL-8 mRNA expression in response to either GSLD or SSD. The fold changes in IL-6 and IL-8 expression were not significantly different between the GSLD and SSD treatments with media alone and those with adenosine, as the values fell within the same error range. These results suggest that 50 μM adenosine may not have been a sufficient dose to antagonize TRPV1 under these conditions.
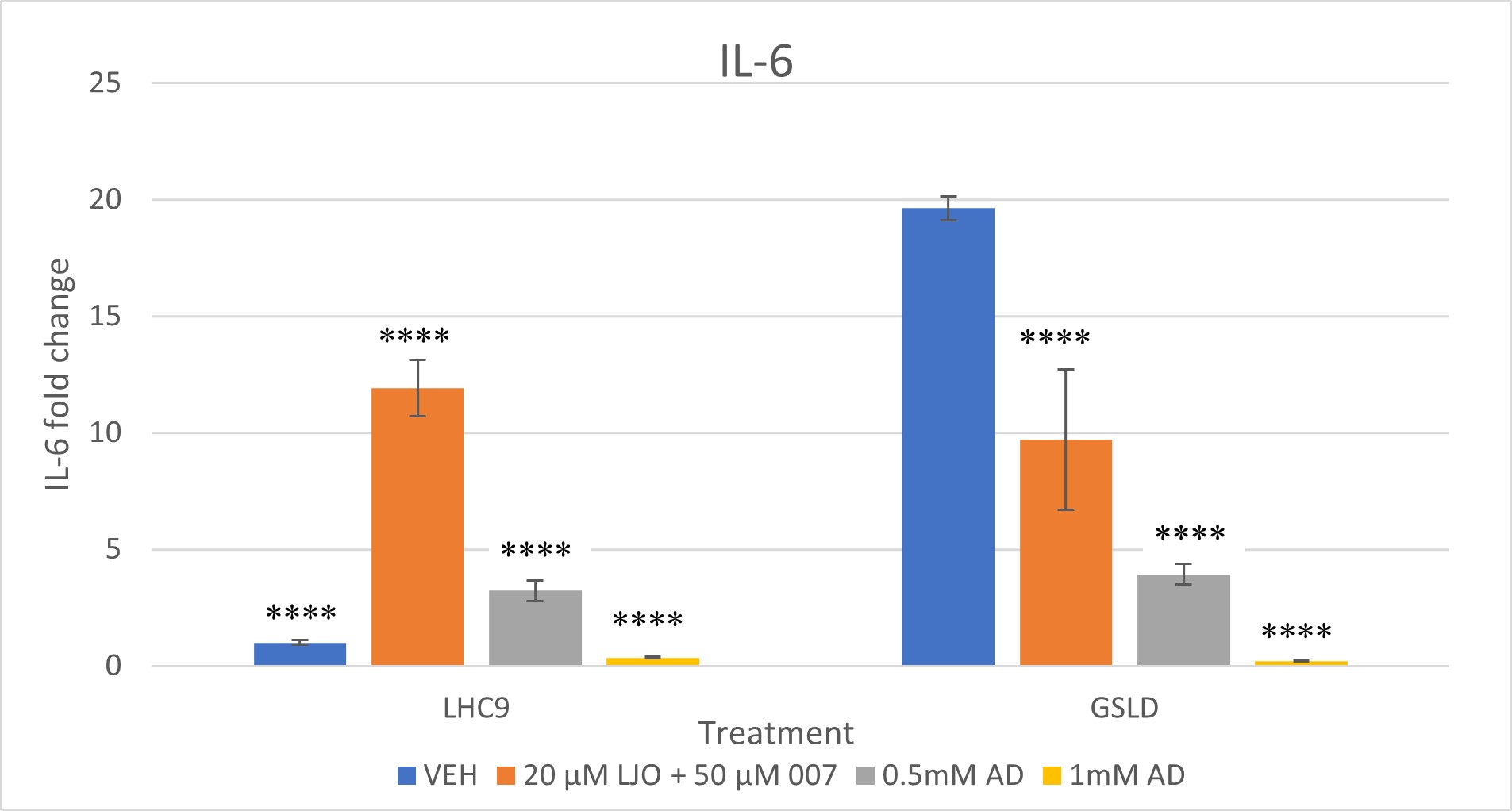
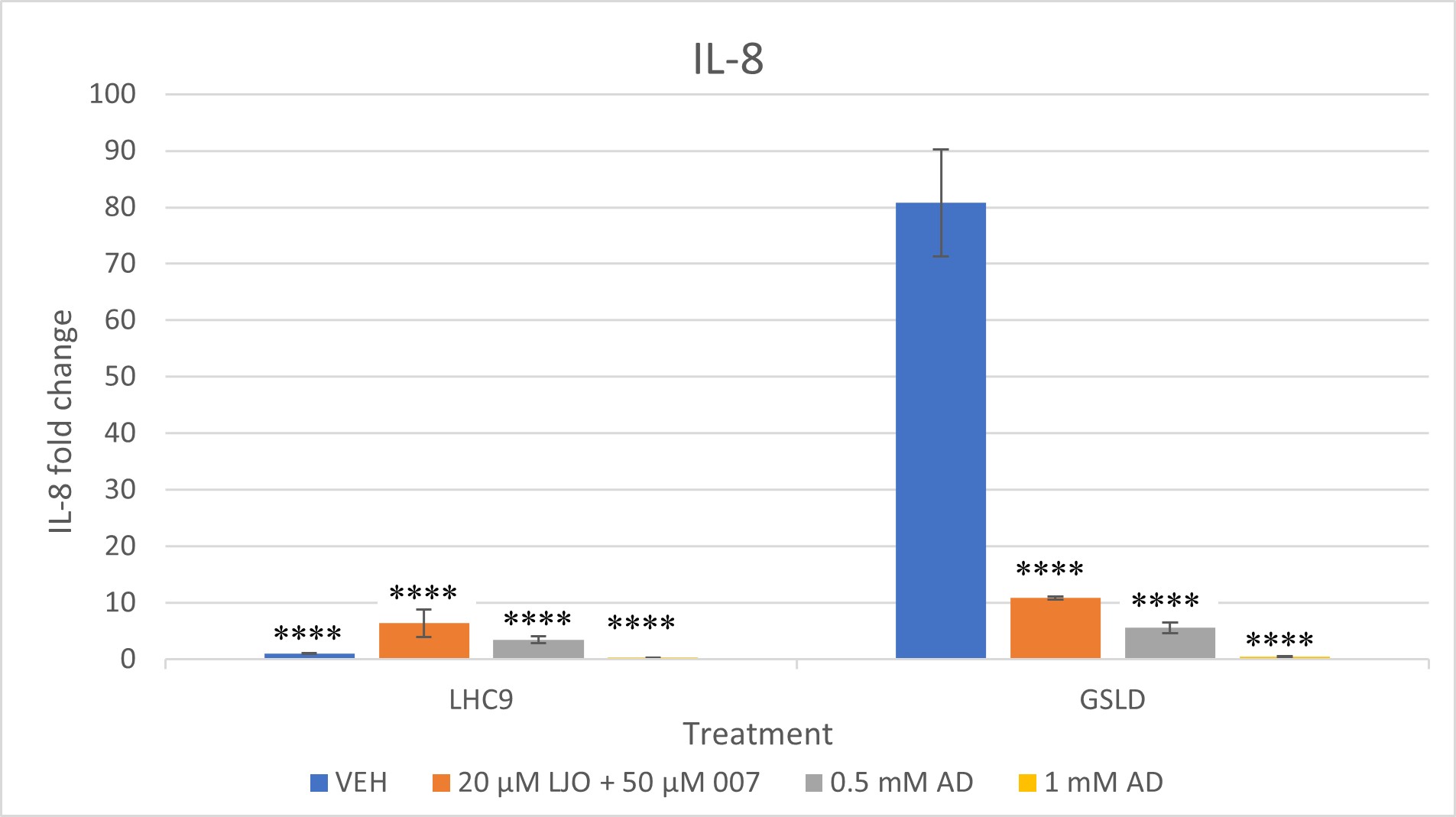
High concentrations of adenosine inhibited IL-6 and IL-8 induction with GSLD. A repeat of the experiment in Figure 4 shows that the hypothesis about adenosine having the potential to inhibit TRPV1 was correct. As the dosage of adenosine was increased from .050 mM to 0.5 mM and 1 mM, inhibition of IL-6 and IL-8 expression mRNA in cells treated with GSLD was observed. The fold change for both IL-6 and IL-8 decreased from about 5 at 0.5 mM to almost 0 at 1.0 mM, with statistically significant differences observed at both 0.5 mM and 1.0 mM concentrations (****, p < 0.0001), showing that the increase in concentration of adenosine is directly proportional to the decrease in gene expression.
Since 007 had previously been shown to not have a strong inhibitory effect on GSLD, the combined inhibitory power of 007 and LJO was tested this time. The combined LJO and 007 treatment successfully inhibited both IL-6 and IL-8 expression in GSLD treated cells with a statistically significant difference when compared to the GSLD control. While IL-6 levels in the LJO + 007 vehicle control were unexpectedly elevated, the treatment group remained significantly different from the GSLD + media control, suggesting that inhibition still occurred despite variability in the control baseline. IL-8 expression showed an even more pronounced reduction, indicating stronger inhibition.
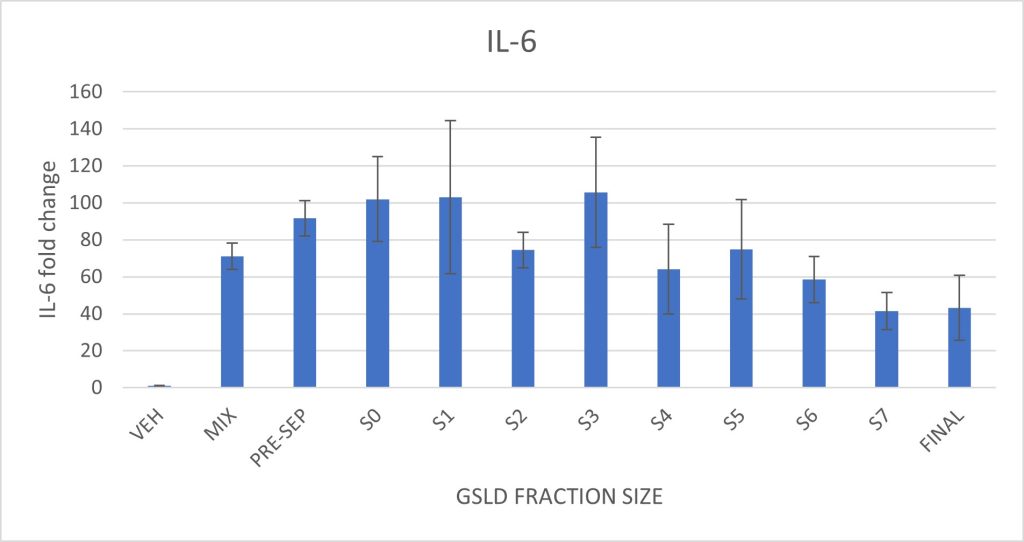
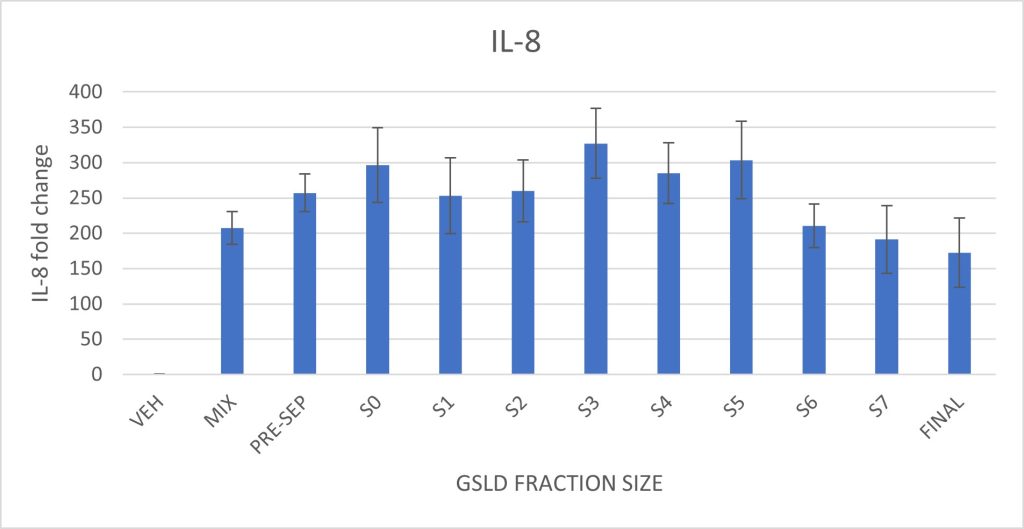
Figure 6. Effect of GSLD particle size on IL-6 and IL-8 expression in BEAS-2B cells. Cells were exposed to size-fractionated GSLD samples, with fractions organized from largest pre-separatory (PRE-SEP) stage to smallest final stage (FINAL). Supplemental Figure 6 shows the particle size distribution and corresponding impactor stages.Statistically significant differences were assessed by comparing each size fraction to the standard GSLD mix described in Methods. There were no statistically significant differences observed.
Particulate matter size had minimal effect on IL-6 and IL-8 expression. A slight decrease in both cytokines was observed in the smallest three size fractions, but these differences were not statistically significant. Larger particle size fractions induced cytokine responses comparable to the full GSLD mixture, indicating that particles throughout the PM10 size range are capable of eliciting an inflammatory response in this model.
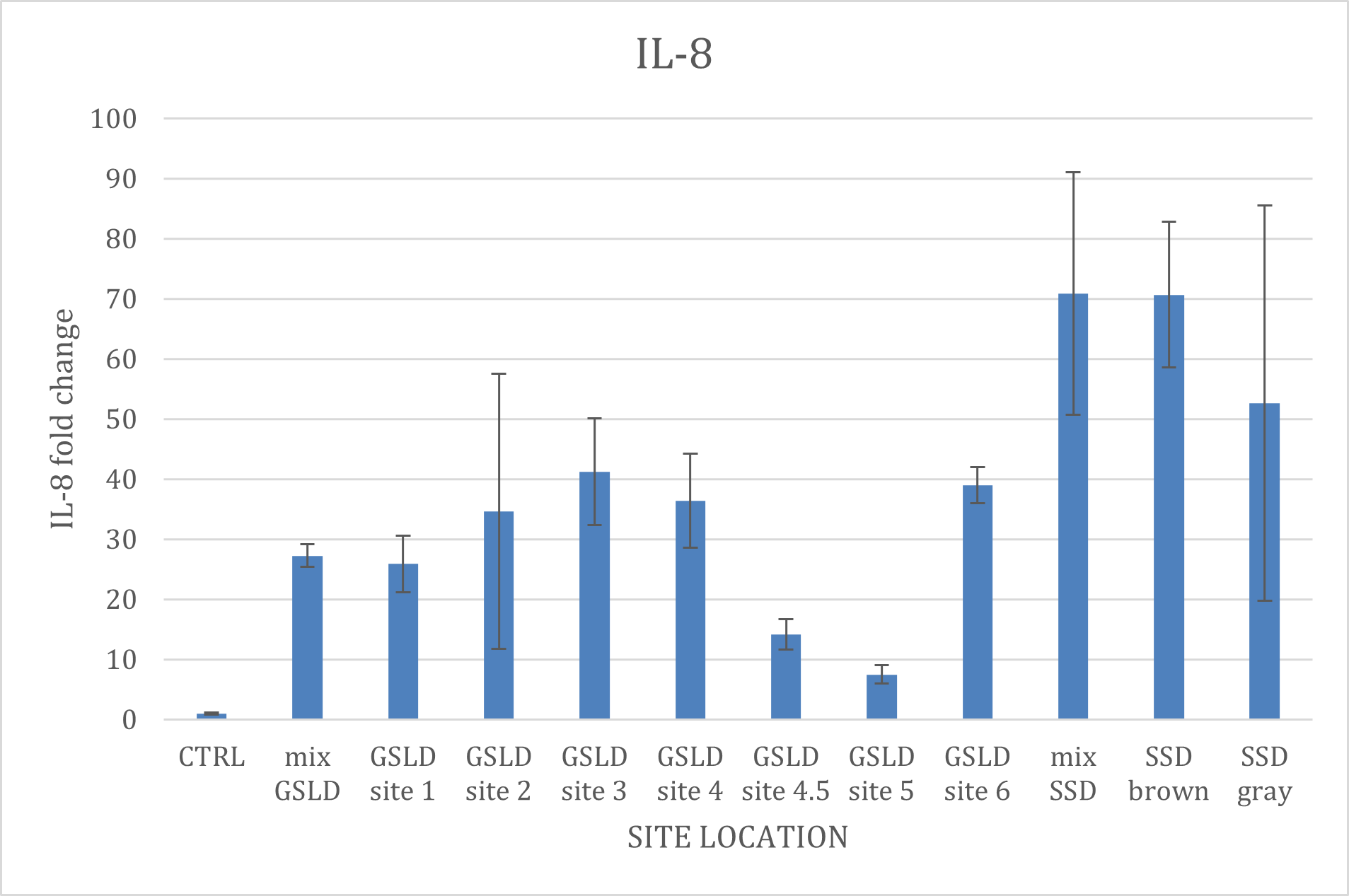
Figure 7. IL-8 expression across different GSLD and SSD collection sites. Due to a technical issue during plate preparation, IL-6 data is not included in this analysis. BEAS-2B cells were exposed to GSLD samples collected from seven distinct sites, as depicted in Supplemental Figure 7. Additionally, data from the SSD mix, along with two site-specific samples were included for comparative analysis. There were no statistically significant differences observed when the GSLD samples were compared to the GSLD mix, and the SSD samples compared to the SSD mix.
Site location also had minimal effects on IL-8 expression. PM2.5 prepared from different GSLD collection sites showed relatively similar pro-inflammatory potency, except for sites 4.5 and 5 which were slightly lower than the others, though not at a statistically significant difference. The GSLD standard mixture, combined from all the sites, was about the average of all the locations, as expected. Since there were only two locations that SSD was collected from, the mixture seemed to more closely match the brown sample, while the gray sample, collected nearest to the existing water level, was less, though the difference was not statistically significant. Despite these differences, we were unable to identify potential causes of the differences in potency through elemental analysis using ICP-MS, indicating a role for other factors. One possibility could be differences in adenosine content, wherein sites with higher adenosine could be less potent. Alternatively, differences in particle suspension properties could impact settling rates and stimulation of cells. Further work is needed in this area.
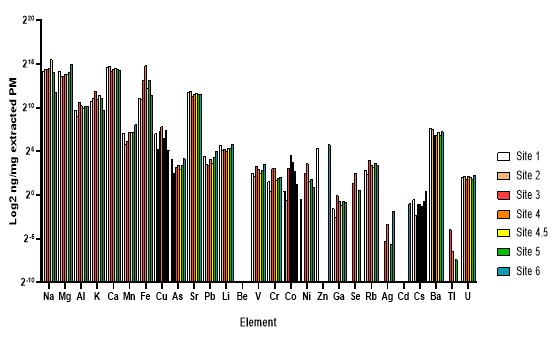
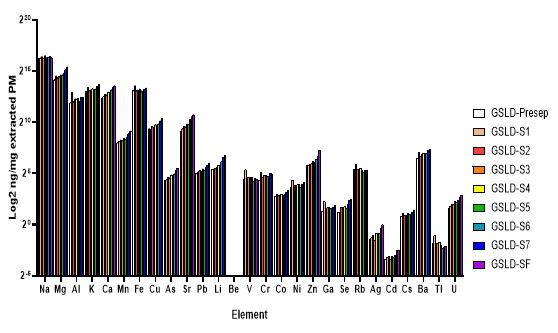
Figure 8. Trace metal content of GSLD samples measured by ICP-MS. Figure 8.A. Comparison across different particle size fractions. Figure 8B. Comparison across GSLD samples from seven distinct collection sites.
Site location and particle size did not differ significantly in trace metal composition. ICP-MS analysis revealed consistent trace metal profiles across both particle size fractions and geographic collection sites of GSLD samples. The absence of significant variation suggests that the elemental composition of GSLD is relatively uniform, regardless of particle size or origin. This uniformity implies that other factors, such as organic components or particle morphology, may play a more substantial role in eliciting biological responses in airway epithelial cells. This supports the qPCR findings, which also showed minimal differences in IL-6 and IL-8 expression across the same variables.
DISCUSSION
This study investigated the pro-inflammatory and cytotoxic effects of particulate matter (PM) collected from the Great Salt Lake (GSLD) and the Salton Sea (SSD). The results show that both types of dust induce calcium flux, reduce cell viability, and upregulate inflammatory cytokine expression, in part via activation of TRPV1 and TRPV3, supporting the hypothesis that saline lake dusts can trigger harmful responses in the airways if sufficient material is inhaled. Results also support the use of BEAS-2B as a reliable airway cell model alongside the previously studied HBEC3-KTs (Cowley et al., 2025).
GSLD induced a more robust calcium flux response compared to SSD, suggesting greater TRP channel activation, while both dusts produced comparable levels of cytokine expression in IL-6 and IL-8 expression, indicating similar downstream inflammatory outcomes. This aligns with the hypothesis that TRP channel activation contributes to GSLD-induced cellular responses. In particular, the magnitude of calcium flux induced by GSLD exceeded that of known TRP agonists such as nonivamide (TRPV1) and carvacrol (TRPA1/TRPV3), indicating that GSLD may strongly activate TRPV1 or other calcium-permeable channels. This could suggest that TRPV1 plays a central role in mediating the observed calcium signaling and potentially downstream inflammatory effects, but that other pathways may also be involved.
The qPCR results for IL-6 and IL-8 mRNA induction further support a TRPV1/V3-dependent mechanism where both GSLD and SSD significantly upregulated IL-8 expression. This response was more strongly attenuated by the TRPV1 antagonist LJO-328, and not as significantly by the TRPV3 antagonist 007 alone, hence why we now use these antagonists as a combination. Interestingly, co-treatments with PM and 007 suppressed IL-6 expression but not IL-8, suggesting that TRPV3 signaling may be more tightly linked to IL-6 production, while IL-8 is predominantly TRPV1-driven. The combination of LJO-328 and 007 proved most effective, supporting previous conclusions from the studies with HBEC-3KTs, indicating possible signaling between TRPV1 and TRPV3 pathways. Unexpectedly, LJO-328+007 alone induced IL-6 even without GSLD, which may reflect off-target effects or a stress response triggered by dual inhibition of calcium-permeable channels, underscoring the delicate balance of homeostatic signaling in airway epithelial cells.
Adenosine was investigated as a potential TRPV1 antagonist based on prior studies showing that its analogs can inhibit TRPV1-mediated calcium flux in HEK-293 cells. While adenosine did not inhibit cytokine induction at 50 μM, as originally tested, subsequent experiments using higher concentrations provided evidence that adenosine can inhibit GSLD induced IL-6 and IL-8 expression in BEAS-2B cells at concentrations of 0.5 mM and 1 mM. This outcome suggests that adenosine may function as a TRPV1 and/or TRPV3 antagonist in airway epithelial cells, pointing to the possibility of adenosine as a therapeutic candidate to mitigate inflammation induced by TRPV1/V3- activating environmental PM such as GSLD. Future studies could also evaluate the efficacy of adenosine as a TRPV1/V3 antagonist in additional lung epithelial cell models, such as the HBEC3-KTs, to determine whether its inhibitory effects extend beyond BEAS-2B cells and using other forms of PM such as SSD.
In experiments comparing GSLD from seven different site locations, only slight variation in cytokine induction was observed. Sites 4.5 and 5 showed mildly reduced IL-8 expression, but the differences were small and not statistically significant. Similarly, when ten different size fractions of GSLD were tested, no clear trends emerged. The smallest three size fractions showed a non-significant decrease in IL-8 expression that may potentially be related to compositional differences or the dose of PM/cell area. What is significant is that the larger particle sizes still induced a cytokine response in IL-6 and IL-8 that was comparable to the GSLD standard mix, suggesting that even PM10 can be harmful to breathe. These findings suggest that while GSLD is overall pro-inflammatory, its biological activity is not strongly dependent on particle size within the PM2.5 range, nor on site-specific composition. Consistent with this, ICP-MS analysis revealed no significant differences in trace metal content across site locations or size fractions, indicating that elemental composition is relatively uniform throughout the sampled dust. Since GSLD is therefore pro-inflammatory regardless of site location and particle size, people who are at risk of exposure to windblown GSLD should also consider PM10 concentrations which tend to be much higher during dust events and of similar potency.
Taken together, this work supports the conclusion that TRPV1 and V3 play a central role in mediating GSLD and SSD-induced inflammation in airway epithelial cells, and that this mechanism is at least partially calcium-dependent. The ability of adenosine to inhibit IL-8 expression at higher concentrations suggests potential for therapeutic intervention. While site location and particle size do not appear to significantly influence inflammatory responses in BEAS-2B cells, this study highlights key mechanistic insights into PM-induced toxicity, particularly the role of TRP channel-mediated calcium signaling. The identification of adenosine as a potential TRPV1/V3 antagonist, offers a promising new avenue for mitigating inflammatory responses to saline lake dusts like GSLD and SSD.
Further studies should validate these findings in additional lung epithelial cell models, such as primary human bronchial epithelial cells (HBECs), to assess their generalizability. In particular, the existing GSLD and SSD samples collected from different site locations and size-fractionated could be repurposed to evaluate whether subtle compositional differences elicit more pronounced responses in primary cells. Finally, characterizing other endogenous components of dust, like adenosine and analogs, or synthetic adenosine mimetics will help clarify roles of this compound and pathway as protective for particulate mixtures.
ACKNOWLEGEMENTS
This work was made possible thanks to the expertise and contributions from the members of the Reilly laboratory, as well as support from the University of Utah Undergraduate Research Opportunity Program. I am grateful to Dr. Naina Phadnis for her support throughout these past two years of my thesis journey and for helping me find an outstanding lab in which I could thrive. I also extend my sincere thanks to Dr. Hye-Joo Kwon at the Utah Asia Campus for her thoughtful feedback and academic guidance during my time abroad while completing this thesis.
I deeply appreciate the mentorship of Drs. Cassandra Deering-Rice, Erin Romero, and Greg Lamb for sharing their expertise in TRP channel biology, dedicating time to teach me various exciting lab techniques to study lung toxicology, and sharing their love of research with me. Their support and patience at every step have been instrumental in fostering my growth, and I will always treasure the knowledge and skills that they generously imparted to me.
Above all, I would like to extend my heartfelt gratitude to Dr. Christopher Reilly for his exceptional guidance and the unwavering support in all opportunities he has provided me with this past year. This would not have been possible without his dedication, understanding, and flexibility to accommodate my personal goals and time constraints. I am profoundly thankful to him and to the Reilly lab for granting me the privilege of completing my Honors Thesis with them, and for their generosity, encouragement, and commitment to my success.
Bibliography
Abbott B., Baxter B.K., Busche K., de Freitas L., Frei R., Gomez T., Karren M.A., Buck R.L., Price J., Frutos S., et al. 2023. Emergency measures needed to rescue Great Salt Lake from ongoing collapse. https://pws.byu.edu/GSL%20report%202023.
Cowley JM, Deering-Rice CE, Lamb JG, et al. 2025. Pro-inflammatory effects of inhaled Great Salt Lake dust particles. Part Fibre Toxicol. 22(2):2. https://doi.org/10.1186/s12989-025-00618-9.
Deering-Rice CE, Nguyen N, Lu Z, Cox JE, Shapiro D, Romero EG, Mitchell VK, Burrell KL, Veranth JM, Reilly CA. 2018. Activation of TRPV3 by wood smoke particles and roles in pneumotoxicity. Chem Res Toxicol. 31(5): 291-301. https://doi.org/10.1021/acs.chemrestox.7b00336.
Deering-Rice CE, Mitchell VK, Romero EG, Abdel Aziz MH, Ryskamp DA, Krizaj D, et al. 2014. Drofenine: a 2-APB analogue with greater selectivity for human TRPV3. Pharmacol Res Perspect. 2(5): e00062. https://doi.org/10.1002/prp2.62.
Frie AL, Dingle JH, Ying SC, Bahreini R. 2017. The effect of a receding saline lake (The Salton Sea) on airborne particulate matter composition. Environ Sci Technol. 51(15):8283-92. https://doi.org/10.1124/mol.117.109959.
Lamb JG, Romero EG, Lu Z, Marcus SK, Peterson HC, Veranth JM, Deering-Rice CE, Reilly CA. 2017. Activation of human transient receptor potential melastatin-8 (TRPM8) by calcium-rich particulate materials and effects on human lung cells. Mol Pharmacol. 92(6):653-664. https://doi.org/10.1124/mol.117.109959.
Marshall JR. 2017. Why emergency physicians should care about the Salton Sea. West J Emerg Med. 18(6):1008-09. https://doi.org/10.5811/westjem.2017.8.36034.
Nguyen ND, Memon TA, Burrell KL, Almestica-Roberts M, Rapp E, Sun L, Scott AF, Rower JE, Deering-Rice CE, Reilly CA. 2020. Transient receptor potential ankyrin-1 and vanilloid-3 differentially regulate endoplasmic reticulum stress and cytotoxicity in human lung epithelial cells after pneumotoxic wood smoke particle exposure. Mol Pharmacol. 98(5):586-97. https://doi.org/10.1124/molpharm.120.000047.
Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, Ramkumar V. 2004. Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci. 24(14):3663- 3671. https://doi.org/10.1523/JNEUROSCI.4773-03.2004.
Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. 2003. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci. 73(1):170-181. https://doi.org/10.1093/toxsci/kfg044.
Tanaka T, Narazaki M, Kishimoto T. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 6(10):a016295. https://doi.org/10.1101/cshperspect.a016295 .
Veranth JM, Kaser EG, Veranth MM, et al. 2007. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 4(2). https://doi.org/10.1186/1743-8977-4-2.
Zhang M, Ma Y, Ye X, et al. 2023. TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Sig Transduct Target Ther. 8:261. https://doi.org/10.1038/s41392-023-01464-x.
APPENDIX
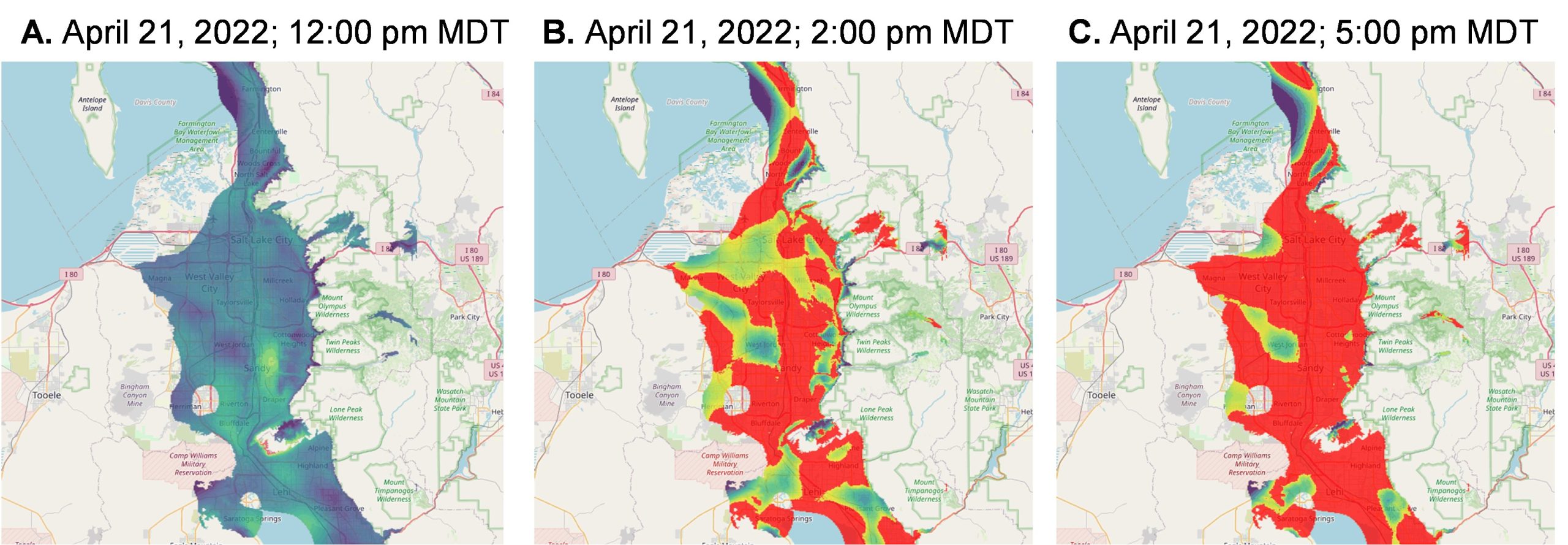
Supplemental Figure 1. Sequential snapshots of a dust event on April 21, 2022. The color scale indicates PM10 concentrations, with purple representing 0–10 μg/m³ and red indicating levels ≥100 μg/m³ (Cowley et al., 2025).
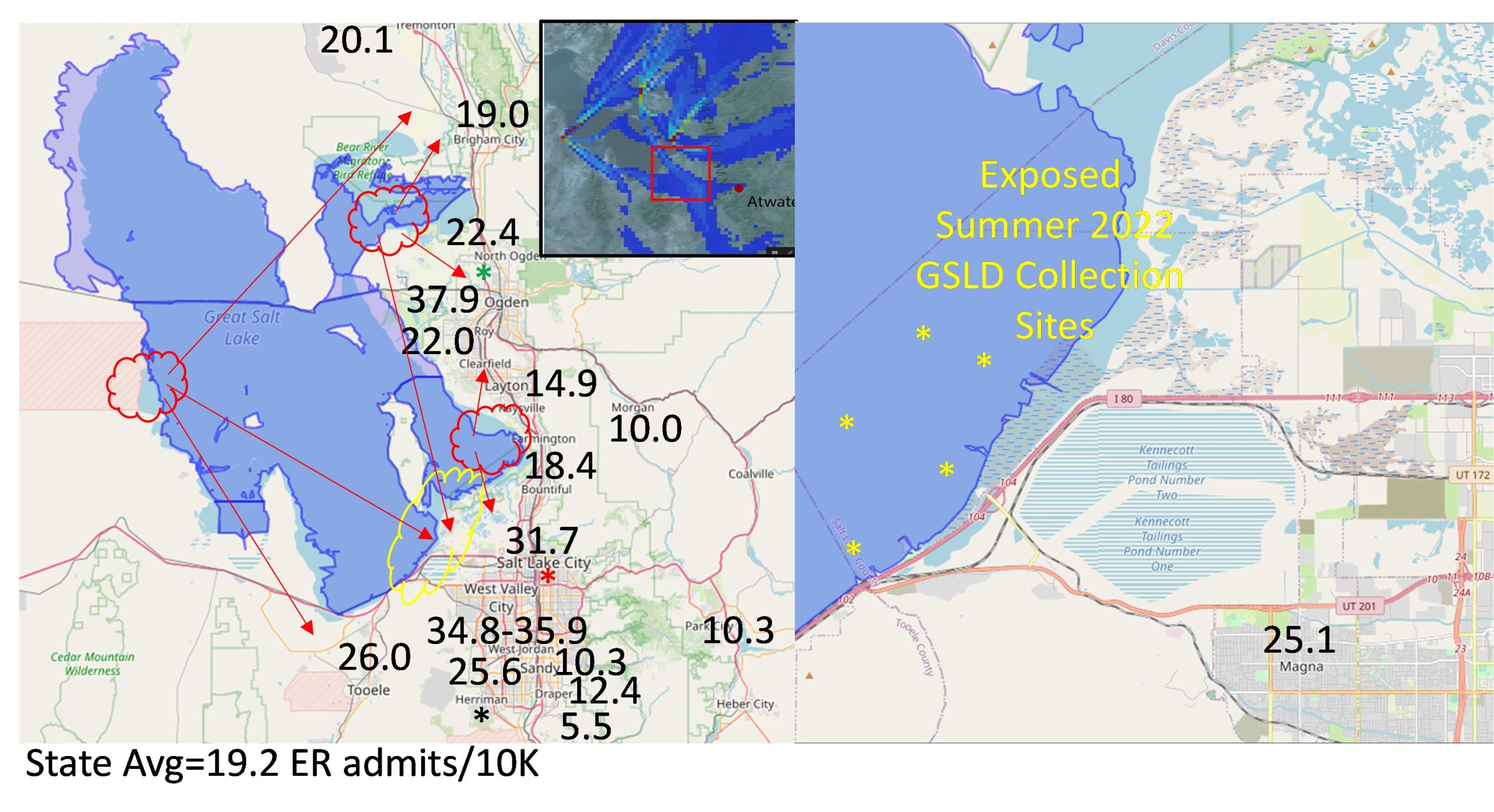 Supplemental Figure 2. Shows number of ER admits/10k people in each region following a dust event, as well as collection sites for GSLD (Unpublished data provided by Dr. Christopher Reilly).
Supplemental Figure 2. Shows number of ER admits/10k people in each region following a dust event, as well as collection sites for GSLD (Unpublished data provided by Dr. Christopher Reilly).
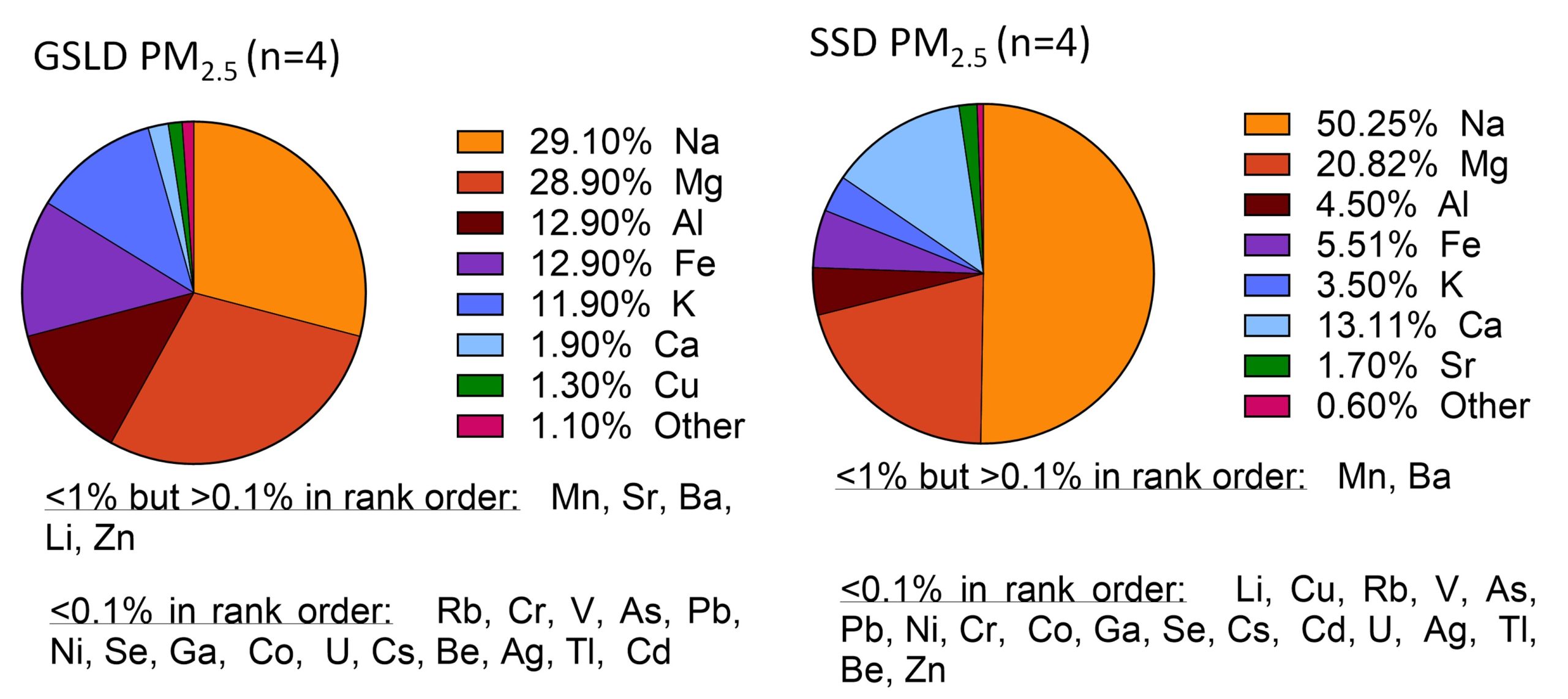 Supplemental Figure 3. Elemental composition of GSLD and SSD PM2.5 samples determined by ICP-MS. Major elements (≥1% by mass) are depicted in a pie chart, while other elements are listed in descending order beneath the respective charts (Data provided by Dr. Christopher Reilly).
Supplemental Figure 3. Elemental composition of GSLD and SSD PM2.5 samples determined by ICP-MS. Major elements (≥1% by mass) are depicted in a pie chart, while other elements are listed in descending order beneath the respective charts (Data provided by Dr. Christopher Reilly).
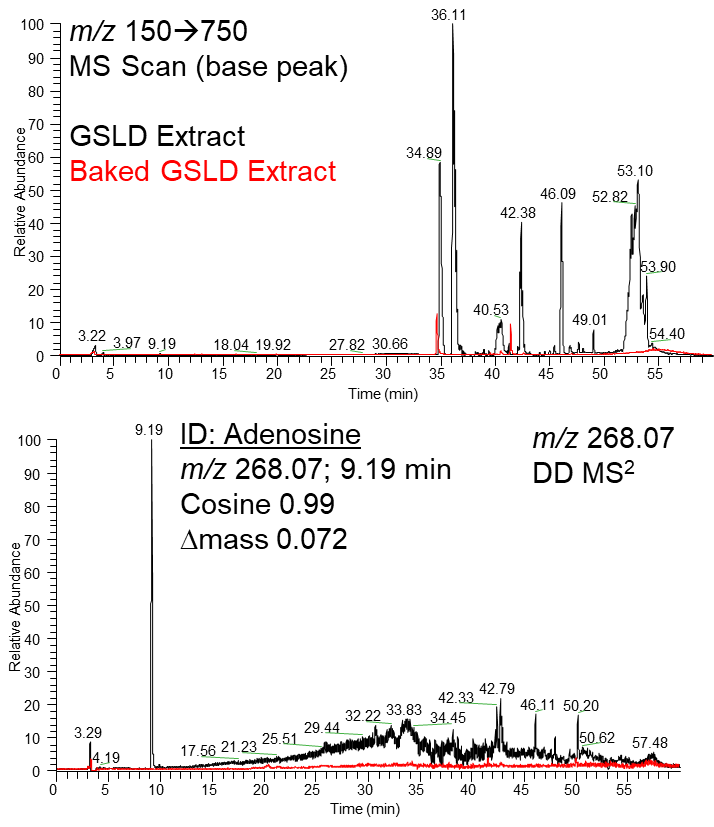
Supplemental Figure 4. LC-MS/MS analysis of baked GSLD extract. Supplemental Figure 4A. Base peak chromatogram (m/z 150–750) showing the most intense ion signal detected at each retention time. Supplemental Figure 4B. A compound detected at 9.19 minutes with m/z 268.07 was identified as adenosine. Its MS/MS fragmentation pattern matched a reference library with a cosine similarity score of 0.99 and a delta mass of 0.072 Da, confirming the presence of adenosine in the sample. (Data provided by Dr. Christopher Reilly).
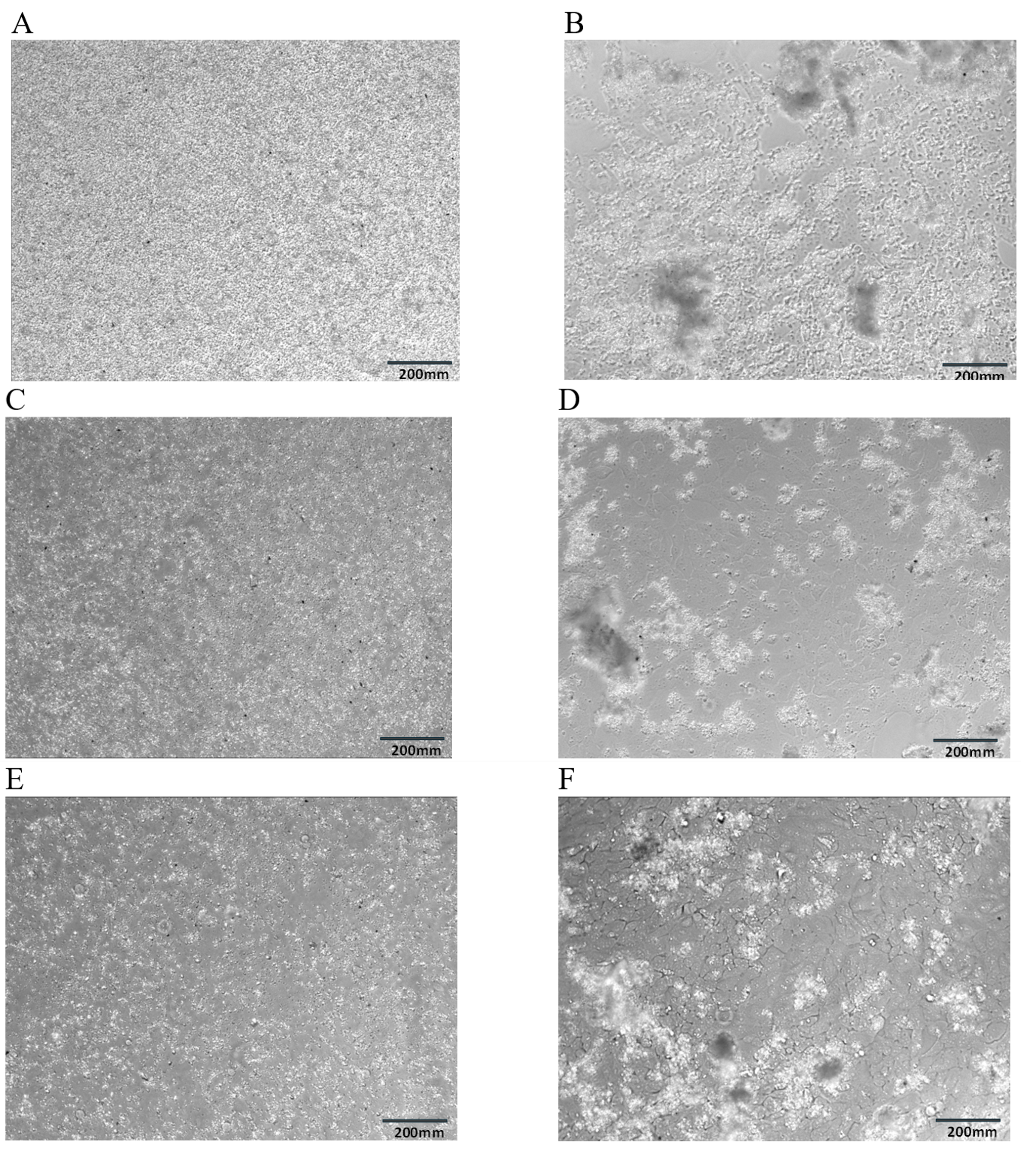
Supplemental Figure 5. Microscope images of BEAS-2B cells treated with GSLD,comparing the GSLD mix with GSLD from two specific sites at initial treatment vs 24 hours later. Supplemental Figure 5A. GSLD mix at 0 hr. Supplemental Figure 5B. GSLD mix at 24 hr. Supplemental Figure 5C. GSLD Site 4.5 at 0 hr. Supplemental Figure 5D. GSLD Site 4.5 at 24 hr. Supplemental Figure 5E. GSLD Site 6 at 0 hr. Supplemental Figure 5F. GSLD Site 6 at 24 hr.
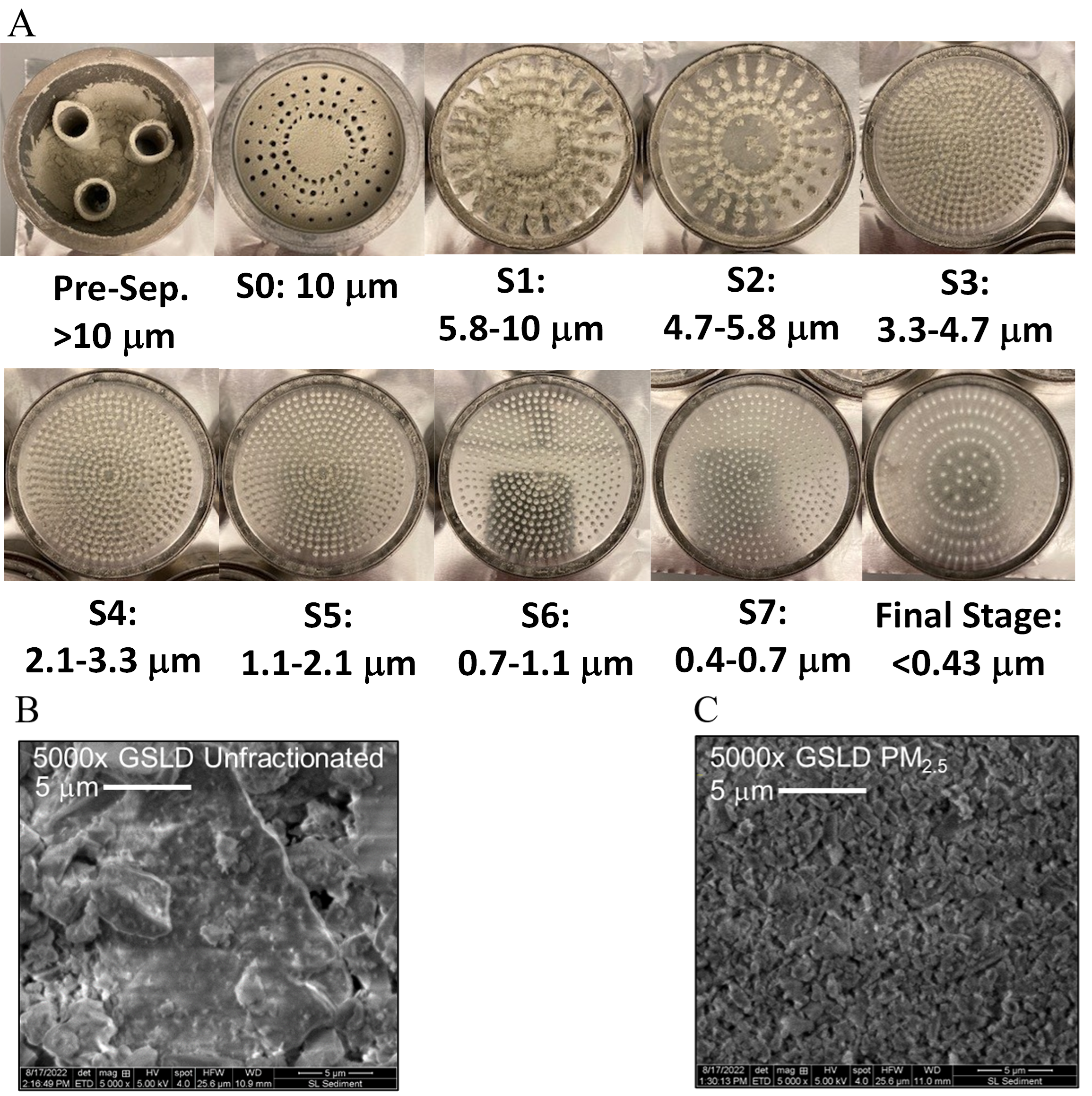
Supplemental Figure 6. Characterization of GSLD particle size distribution and morphology. Figure 6A. Stages of the Andersen Cascade Impactor and corresponding aerodynamic diameter range (provided by Dr. Christopher Reilly). GSLD deposition across each stage highlights the distribution of particles by size. Supplemental Figure B. Electron micrographs (5000X) of unfractionated GSLD. Supplemental Figure C. Electron micrographs (5000X) of GSLD PM2.5 (Cowley et al., 2025).
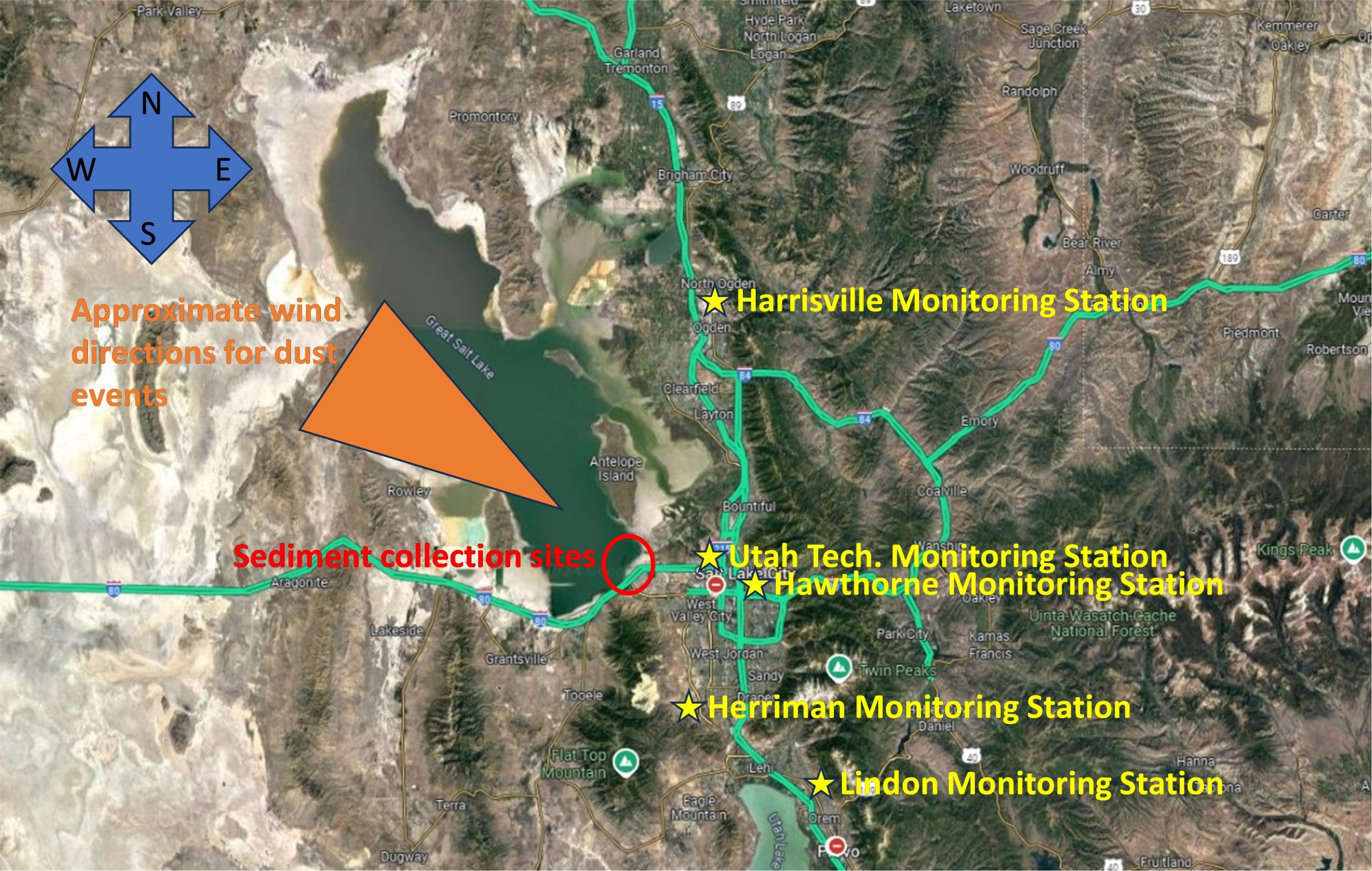
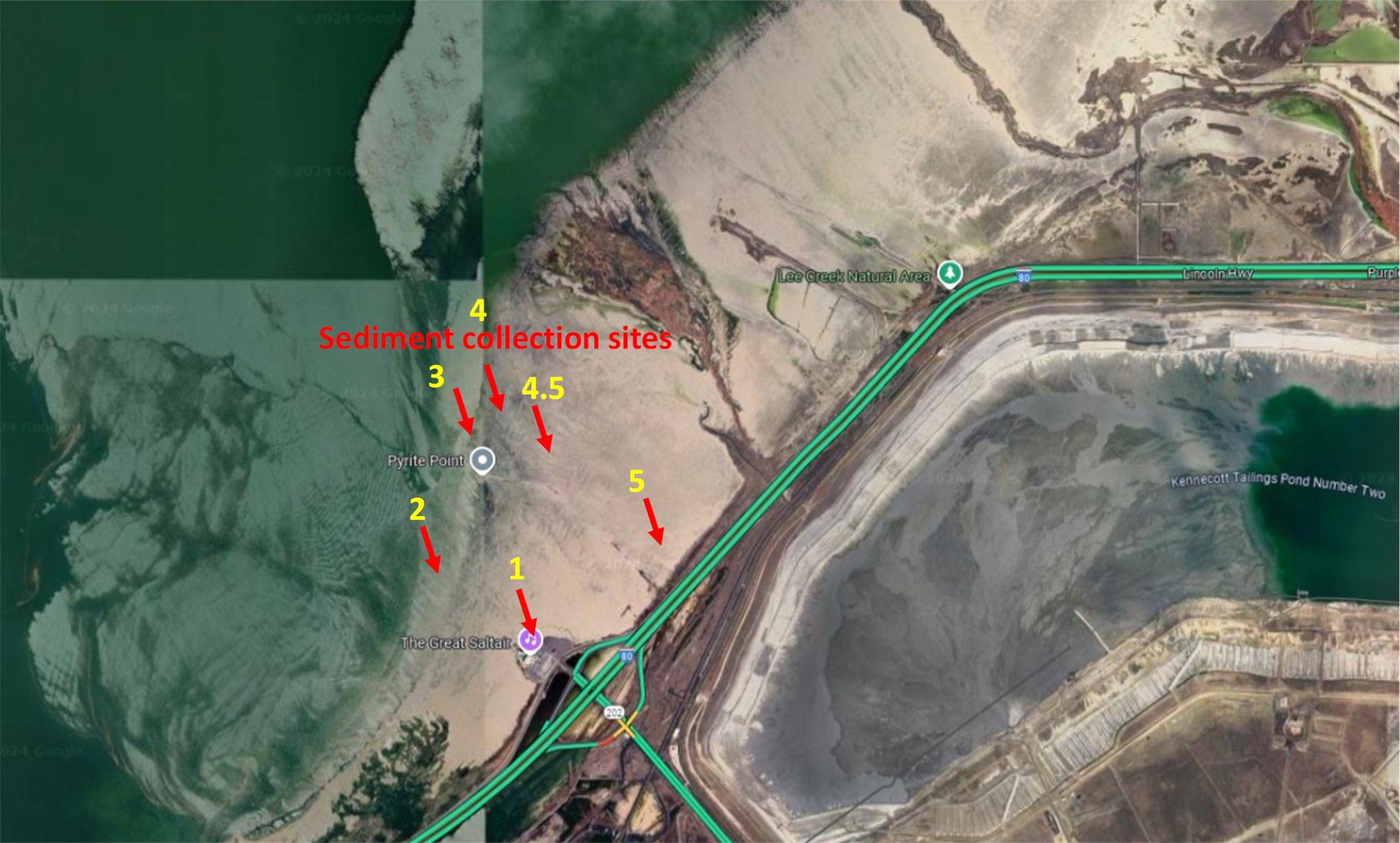
Supplemental Figure 7A. Great Salt Lake sediment collection sites, locations of monitoring stations, and winds direction during highlighted dust events. Supplemental Figure 7B. Zoomed map showing the locations of GSLD sample collection sites used in this study (Cowley et al., 2025). Only the first six collection sites are shown in this image. Site 6 is farther north, located near Antelope Island in Syracuse, Utah, GPS coordinates: 41.048889, – 112.113611 (provided by Dr. Christopher Reilly).
Media Attributions
- Figure 1A
- Figure 1B
- Figure 2A
- Figure 2B
- Figure 2C
- Figure 2D
- Figure 2E
- Figure 3
- Figure 4A
- Figure 4B
- Figure 5A
- Figure 5B
- Figure 6A
- Figure 6B
- Figure 7
- Figure 8A
- Figure 8B
- Supplemental Figure 1
- Supplemental Figure 2
- Supplemental Figure 3
- Supplemental Figure 5
- Supplemental Figure 6
- Supplemental Figure 7A
- Supplemental Figure 7B

