College of Health
46 Effects of Nitric Oxide Gel on Collagen Organization After AVF Creation
Kevin Pan
Faculty Mentor: Yan-ting Shiu (Internal Medicine, University of Utah)
Introduction
Chronic Kidney Disease (CKD) is a significant health concern, affecting approximately 37 million Americans, or 15 percent of the U.S. adult population [1]. The kidneys, responsible for filtering waste and regulating essential functions like blood pressure and electrolyte balance, are vital to maintaining bodily equilibrium. CKD cannot be cured, and the most effective treatment is renal replacement therapy (RRT). However, the high demand for kidney donors presents significant challenges and most patients will use dialysis instead. For CKD patients undergoing dialysis, the arteriovenous fistula (AVF) is the gold standard for vascular access. A successful AVF relies on the proper maturation of the vessel and depends on outward remodeling to allow adequate blood flow. Following the creation of AVFs, there is an increased synthesis of collagen, initiating a gradual and irreversible process known as fibrosis. The arrangement of collagen pattern in the extracellular matrix (ECM), plays a vital role in the remodeling process.
Nitric oxide (NO) is known to facilitate outward ECM remodeling by promoting vasodilation [2], making it a potential therapeutic solution for AVF maturation. Despite the known benefits of NO on vascular remodeling improvement, it remains unclear how NO impacts the arrangement of collagen in the medial layer of the AVF. In vascular injury models, NO has also been shown to modulate ECM composition and suppress excessive collagen deposition [3]. As collagen alignment and integrity influence vessel compliance and patency, understanding how NO effects collagen organization could offer insight into strategies for improving AVF maturation and long-term functionality in dialysis patients. This led to the hypothesis that NO treatment would not alter collagen fiber patterns, and that AVFs treated with NO gel would exhibit similar collagen organization to non-AVF vessels.
The objective of this study is to examine the influence of NO on collagen arrangement within the media layer of the anastomosis site in AVFs using a porcine model. An NO-releasing gel was applied to the anastomosis sites of pig AVFs between internal of the jugular vein to the carotid artery. It was then immediately injected with perivascular application of NO gel after the AVF creation. AVF image was obtained using second harmonic generation microcopy (SHG). To determine the impact of NO gel on AVF, collagen arrangement within the media layer was analyzed using ImageJ and MATLAB. Comparative image analysis between NO-treated and untreated groups was used to evaluate the effect of NO on collagen fiber organization and patterning.
Methods
The studies utilized both male and female 50kg Yorkshire pigs (Snyder Farms, Southside, AL). Subjects were split into four groups: [NO-treated AVF, Non-AVF, NO-treated Non AVF, and AVF]. The animal was anesthetized with isoflurane, and the skin in the ventral neck area was shaved and disinfected. The pig AVFs were then created by connecting the jugular vein to the carotid artery. The animal was then positioned supine on a heating blanket to maintain a body temperature of 37°C throughout the procedure. After completion of the AVF, the NO-releasing nano-matrix gel was applied over the AVF anastomosis site, followed by closure of the skin with suture. After surgery, the animal was monitored for 35 days before being sacrificed for tissue collection using SHG.
Qualitative Analysis
After acquiring the image, qualitative analysis of different types of collagen patterns in the media layers is performed. These patterns are described by comparing the collagen fibers alignment within the circumferential vessel section in comparison to the lumen. The terms used to describe the collagen pattern are depicted in Figure 1.
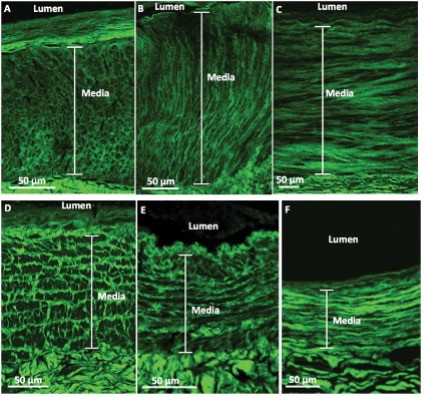
Figure 1: Representative second-harmonic-generation (SHG) collagen patterns [4].
- Honeycomb: Collagen fibers resemble a web-like structure.
- Perpendicular: Collagen fibers that run vertically with respect to the vessel’s lumen
- Parallel: Collagen fibers that run horizontally with respect to the vessel’s lumen
- Railroad Track: Collagen fibers run in a mixture of parallel and perpendicular that makes look like a railroad track with respect to the lumen.
- Micro-Perpendicular Macro-Parallel (MPMP): When the image is zoomed out, the collagen fibers appear to be parallel with respect to the lumen, but once zoomed in, the collagen is made up of thick looking perpendicular fibers.
- Mixed: Two or more of the distinct patterns
Quantitative Analysis
Numerical analyses were conducted using ImageJ version 1.54c (National Bioimaging Solutions, Boston, MA) and MATLAB version R2023a (MathWorks Inc., Natick, MA) to evaluate collagen organization within the medial layer of the vessel. Images were first aligned with the vessel lumen at 180 degrees using a PowerPoint template to determine the appropriate rotation angle. This angle was then applied in ImageJ to properly orient each image and regions of interest (ROIs) were selected specifically from the medial layer for further analysis. After isolating medial ROIs, images were converted to 16-bit grayscale and analyzed using the ImageJ’s OrientationJ plugin to calculate dominant fiber orientation The Dominant Direction Angle represents the average alignment of collagen fibers with respect to the lumen. The Anisotropy Index (AI), ranging from 0 to 1, quantifies fiber alignment—where 0 indicates random orientation and 1 indicates perfect alignment. For each image, the OrientationJ “Dominant Direction” and “Measure” features were used to ensure consistency in angle and AI values. The selected regions were then resized to square dimensions (128×128 or 256×256 pixels) and exported as JPEGs for MATLAB analysis. MATLAB was used to verify AI values using custom written program from the Shiu Lab database. These metrics were then used to calculate the Fiber Configuration Index (FCI) using the equation: FCI = sin(angle in radians) × AI, which captures both fiber directionality and degree of alignment.
Results
Qualitative Analysis
The most common medial collagen fiber patterns observed in the NO-treated AVF, non-AVF, and NO- treated non-AVF groups were predominantly parallel, with fibers aligned along the circumference of the lumen (Figure 4). In contrast, the untreated AVF group exhibited more variability, with collagen fibers displaying a mix of perpendicular, and random alignment patterns, making random orientation more common in this group (Figure 1).
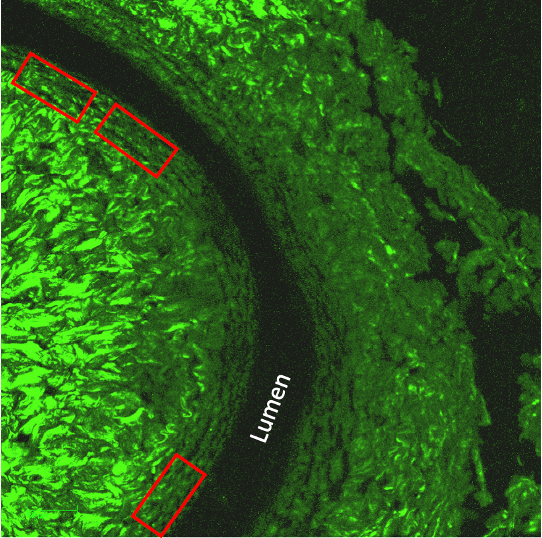
Figure 2: The first two collagen images show a Nitric Oxide sample with non-AVF (first) and AVF (second) regions exhibiting parallel collagen patterns. Similarly, the last two images display parallel collagen patterns observed in non-AVF (third) and AVF (fourth) regions treated with the NO gel.
Quantitative Analysis
Quantitative analysis of the Anisotropy Index (AI) revealed differences in collagen fiber alignment across treatment and anatomical location groups (figure 6). The Control Contralateral group exhibited the highest variability (SD = 0.162), followed by the Control Past Anastomosis group (SD = 0.150). In comparison, both NO Gel-treated groups demonstrated lower variability, with the NO Gel Contralateral group showing the lowest SD (0.115), and the NO Gel Past Anastomosis group showing moderate variability (SD = 0.133). These findings suggest that NO Gel treatment may contribute to more consistent collagen fiber alignment, regardless of anatomical location. The visualization further highlights the overall effect of NO treatment in reducing the variability of ECM organization compared to untreated controls.
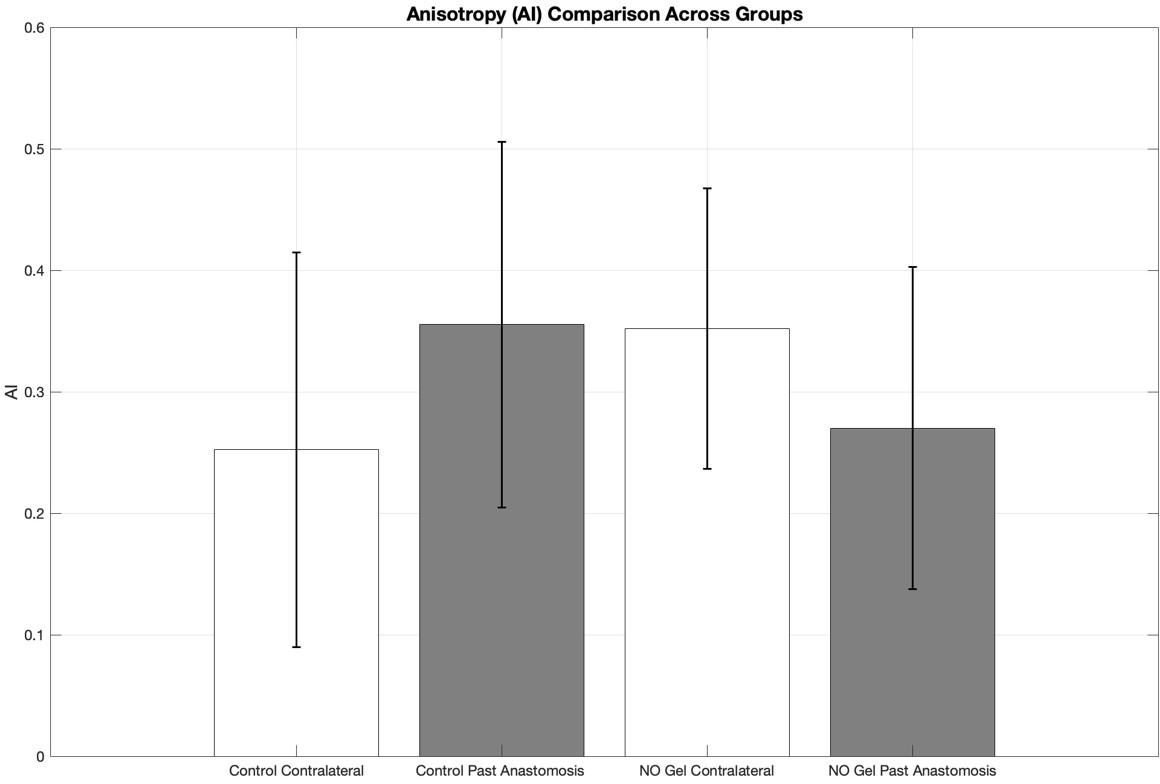
Figure 3: Bar graph illustrating the mean Anisotropy Index (AI) ± standard deviation (SD) for four experimental groups: Control Contralateral, Control Past Anastomosis, NO Gel Contralateral, and NO Gel Past Anastomosis.
Orientation Angle (OA) variability was assessed across all experimental groups to evaluate the consistency of collagen fiber direction. The Control Contralateral group exhibited the greatest variability (SD = 32.4), followed by the Control Past Anastomosis group (SD = 19.3). The NO Gel Past Anastomosis group showed a similar level of variability (SD = 19.2), while the NO Gel Contralateral group demonstrated the lowest variability (SD = 5.98). These results suggest that NO Gel treatment, particularly in the contralateral region, may lead to more uniform collagen fiber alignment. The comparison highlights distinct differences in OA variability between treated and untreated vessels, as well as between anatomical sites.
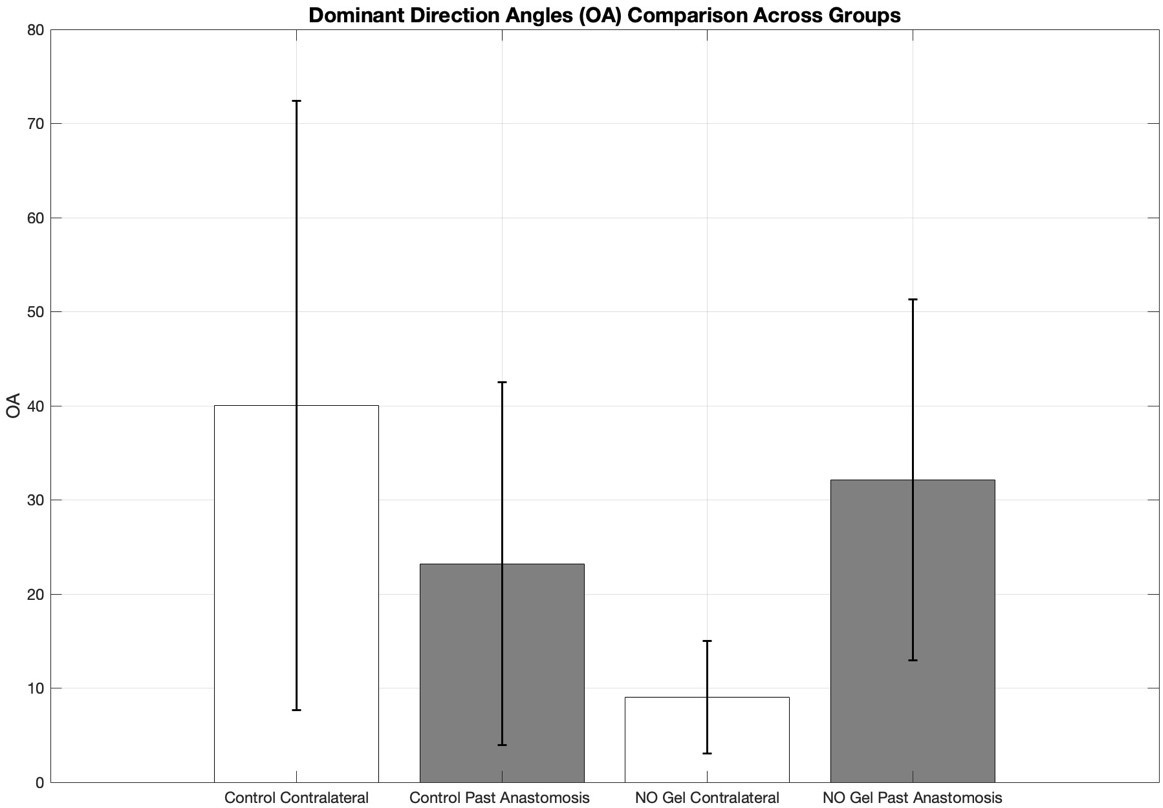
Figure 4: Bar graph showing the mean dominant direction angles (OA) ± standard deviation (SD) for four experimental groups: Control Contralateral, Control Past Anastomosis, NO Gel Contralateral, and NO Gel Past Anastomosis
Variability in the Fiber Configuration Index (FCI) was compared across treatment and anatomical location groups (figure 8). The Control Contralateral and Control Past Anastomosis groups showed the greatest variability, with SD values of 0.14 and 0.14, respectively. In contrast, the NO Gel Contralateral group exhibited the lowest variability (SD = 0.052), and the NO Gel Past Anastomosis group showed moderate variability (SD = 0.097). These results suggest that NO Gel treatment, particularly in the contralateral vessels, may contribute to more consistent collagen organization within the vessel wall. This pattern supports the potential role of NO in stabilizing ECM architecture compared to untreated controls.
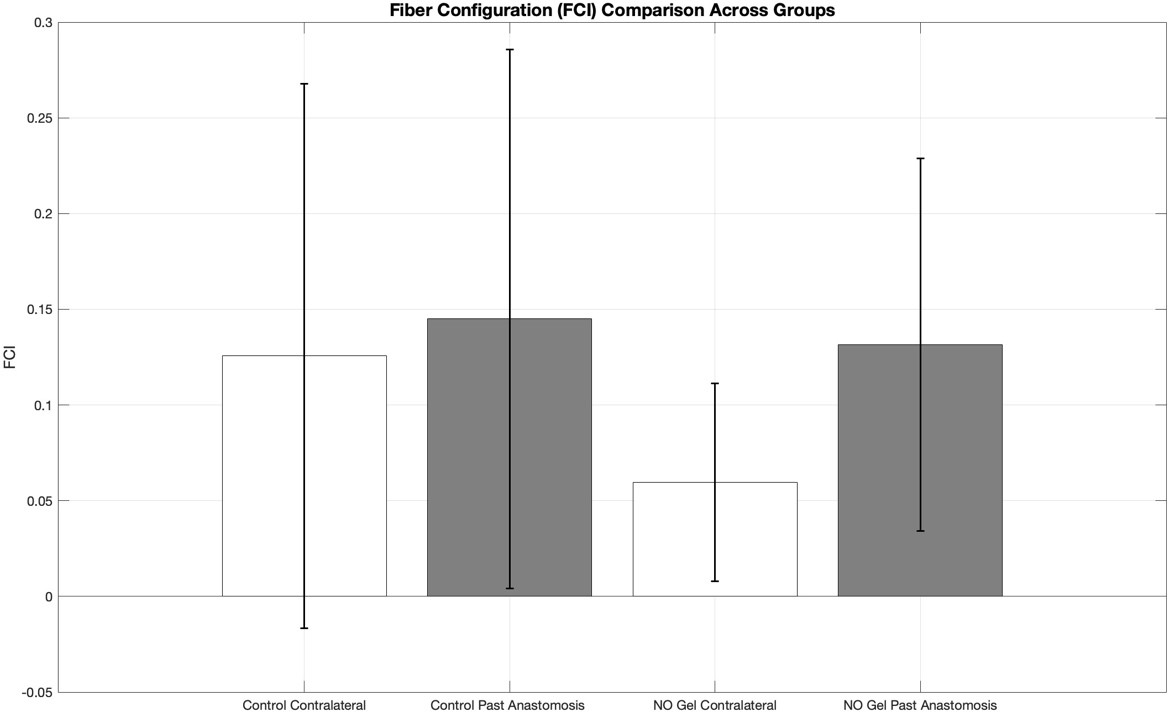
Figure 5: Bar graph showing the mean fiber configuration index (FCI) ± standard deviation (SD) for four experimental groups: Control Contralateral, Control Past Anastomosis, NO Gel Contralateral, and NO Gel Past Anastomosis.
Two-Way ANOVA Test
To assess how nitric oxide (NO) treatment and anatomical location influence collagen organization, a two-way ANOVA was conducted for three key metrics: Anisotropy Index (AI), Orientation Angle (OA), and Fiber Configuration Index (FCI) (Table 1). For AI, no significant main effects were observed for either treatment or location. However, a significant interaction between treatment and location was detected, suggesting that the effect of NO Gel on fiber alignment may vary depending on anatomical site. In contrast, OA showed a significant main effect of NO Gel treatment, while the location effect was not significant Additionally, a highly significant interaction was observed, indicating that NO Gel meaningfully influences collagen fiber orientation in a location-dependent manner. For FCI, there was no significant main effect of NO treatment though a significant main effect of anatomical location was observed and the interaction between the two factors was not significant Collectively, these results suggest that NO treatment primarily affects the direction and alignment of collagen fibers, particularly when anatomical context is taken into account, while location alone may play a more consistent role in influencing fiber configuration.
|
Metric |
Main Effect: NO Gel (F, p) |
Main Effect: Control (F, p) |
Interaction (F, p) |
|
Anisotropy Index (AI) |
F = 0.08, p = 0.78 |
F = 0.16, p = 0.69 |
F = 13, p = 0.0005 |
|
Orientation Angle (OA) |
F = 8.0, p = 0.0055 |
F = 0.65, p = 0.42 |
F = 26, p < 0.001 |
|
Fiber Configuration Index (FCI) |
F = 3.6, p = 0.059 |
F = 4.8, p = 0.031 |
F = 1.6, p = 0.21 |
Table 1: This table presents the results of Two-Way ANOVA analyses examining the effects of treatment (NO Gel vs. Control), anatomical location (Contralateral vs. Past Anastomosis), and their interaction on three quantitative metrics of collagen organization: Anisotropy Index (AI), Orientation Angle (OA), and Fiber Configuration Index (FCI).
Discussion
Chronic kidney disease (CKD) is a significant global issue, leading to an increased demand for hemodialysis. However, the success of dialysis is heavily reliant on properly matured arteriovenous fistulas (AVFs), which often fail due to inward remodeling. Addressing this issue is crucial for improving vascular access for CKD patients that need dialysis. This study builds on previous studies showing that nitric oxide effectively enhances lumen size in AVFs [8]. This study aimed to investigate whether nitric oxide (NO) gel application promotes favorable collagen patterns within the medial layer of AVFs while preserving collagen pattern organization. AVFs were created in a porcine model and treated with NO-releasing compounds to evaluate collagen using MATLAB and ImageJ. The finding that NO gel does not interfere with the natural alignment of collagen fibers suggests that it promotes outward remodeling without disrupting extracellular matrix (ECM) structure. This is further supported by qualitative observations, which showed that samples from the NO-treated AVF, non-AVF, and NO- treated non-AVF groups predominantly exhibited organized parallel collagen patterns. In contrast, untreated AVF samples displayed more disorganized and random collagen fiber arrangements.
Together, these results indicate that NO gel may help preserve native collagen architecture, which is critical for maintaining vessel flexibility and promoting successful AVF maturation.
The combined statistical and visual analysis of collagen structure offers important insights into the role of NO gel in AVF remodeling. Quantitative analysis showed no significant main effect of NO treatment on Anisotropy Index (AI) or Fiber Configuration Index (FCI), and although Orientation Angle (OA) did show a treatment effect, this primarily reflected location-dependent variability rather than structural disruption (Table 1). Importantly, a significant interaction between NO treatment and anatomical location was observed for both AI and OA, indicating that NO gel may influence collagen organization in a context-specific manner. Complementing these findings, qualitative analysis further confirmed that NO-treated AVFs, non-AVFs, and NO-treated non-AVFs exhibited well-organized, predominantly parallel collagen fiber patterns, while untreated AVF samples often displayed random or disorganized arrangements. These consistent patterns suggest that NO gel preserves collagen structural integrity while promoting outward remodeling. Together, these findings highlight the potential of NO gel as a supportive agent in AVF development by maintaining healthy ECM organization without introducing structural disruption.
This study provides valuable insights into the ability of NO to promote favorable collagen alignment and enhance AVF maturation. These findings support the therapeutic potential of NO gel in enhancing AVF maturation by preserving collagen organization during vascular remodeling. By maintaining the structural integrity of the extracellular matrix without disrupting fiber alignment, NO gel may contribute to more reliable AVF development. This positions nitric oxide as a promising agent in the advancement of vascular access therapies and may help improve outcomes for patients undergoing hemodialysis. This approach could change how AVFs are managed and prepared for patients. If it works in clinical settings, it may help make dialysis access more reliable. It could also lower healthcare costs by reducing the number of AVF failures. Most importantly, it could improve treatment outcomes for millions of dialysis patients by making access last longer, lowering complication rates, and supporting better kidney health.
References
[1] “Kidney Disease Statistics for the United States | NIDDK,” National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/healthstatistics/kidney-disease#:~:text=Chronic%20kidney%20disease%20(CKD)%20affects
[2] Somarathna, Maheshika, et al. “Nitric Oxide Releasing Nanomatrix Gel Treatment Inhibits Venous Intimal Hyperplasia and Improves Vascular Remodeling in a Rodent Arteriovenous Fistula.” Biomaterials, vol. 280, 1 Jan. 2022, p. 121254, pubmed.ncbi.nlm.nih.gov/34836683/, https://doi.org/10.1016/j.biomaterials.2021.121254.
[3] T. Vacek, S. Rahman, S. Yu, D. Neamtu, S. Givimani, and S. Tyagi, “Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms,” Vascular Health and Risk Management, p. 173, Feb. 2015, doi: https://doi.org/10.2147/vhrm.s68415.
[4] Y.-T. Shiu, J. I. Rotmans, W. J. Geelhoed, D. B. Pike, and T. Lee, “Arteriovenous conduits for
hemodialysis: how to better modulate the pathophysiological vascular response to optimize vascular access durability,” American Journal of Physiology-renal Physiology, vol. 316, no. 5, pp. F794–F806, May 2019, doi: https://doi.org/10.1152/ajprenal.00440.2018.

