Spencer Fox Eccles School of Medicine
67 Placental Lipid Droplet Accumulation in Uteroplacental Insufficiency: Preferential Trapping of DHA- Containing Triglycerides
Kiera Kearns; Lisa Joss-Moore; Amara Finch; Wesley Chidester; Bridget Raymundo; James Cox; and Alan Maschek
Faculty Mentor: Lisa Joss-Moore (Pediatrics, University of Utah)
Background
Fetal acquisition of long-chain polyunsaturated fatty acids or LCPUFA is critical for the appropriate development of organs including the brain and lung, this is particularly true of the omega 3 fatty acid DHA, and the omega 6 fatty acid, ARA. The organ responsible for conveying this lipid transfer to the fetus is the placenta. In uncomplicated pregnancies, the placenta processes maternal circulating lipids to free fatty acids, which are transported into cytotrophoblasts and syncytiotrophoblasts at the maternal-fetal interface of the placenta. These lipids can then be transported to the fetus, in a process that results in biomagnification of the LCPUFA, particularly for the fatty acids DHA and ARA, or lipids can be metabolized locally undergoing catabolic or anabolic metabolism. Our study focused on anabolic lipid metabolism, specifically the synthesis and storage of lipids.
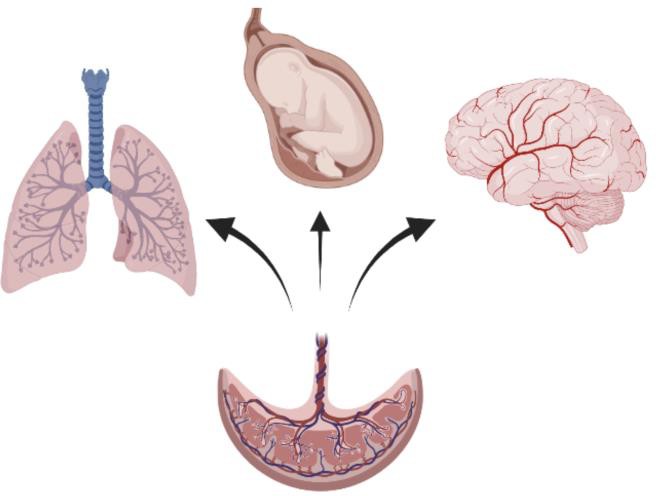
Figure 1: The placenta facilitates lipid transfer to the fetus to support development of organs including the lung and brain.
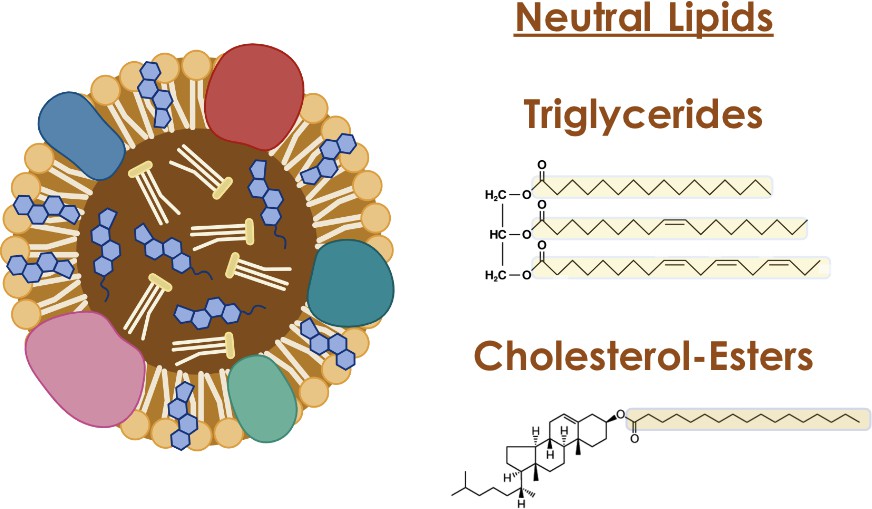
Lipid storage in most tissues, including the placenta, occurs within lipid droplets, organelles with a central hydrophobic core of neutral lipids surrounded by a single layer of phospholipids and proteins. Lipid droplets are major regulators of cellular metabolism – in that they can act as hubs that coordinate pathways of lipid uptake, distribution, storage and use within the cell1. Some of these activities are accomplished by lipid droplets interacting with other organelles like mitochondria, allowing for coordinated lipid metabolism. The lipids in lipid droplets are mainly neutral lipids such as triglycerides and cholesterol esters, with 3 fatty acids incorporated into a triglyceride and one fatty acid into a cholesterol ester.
Pathologic conditions of pregnancy, particularly those with a hypoxic component such as uteroplacental insufficiency or UPI, result in increased placental lipid accumulation and lipid droplet formation2. Dr Yoel Sadovsky’s3 group showed by Oil Red O stain, which binds lipids, that trophoblasts cultured in standard and hypoxic conditions retain lipids differently (Figure 3). Lipid droplets showed to be significantly increased in hypoxic conditions compared to the standard counterparts. Using a rat model, our group has previously demonstrated that UPI increases lipid droplet accumulation in the placenta, with worse effects in the male placenta (Figure 4). We also showed that term fetuses from UPI pregnancies have reduced circulating DHA and ARA fatty acids. While we have demonstrated lipid droplet accumulation in UPI, whether DHA and ARA fatty acids are being trapped in lipid droplets in UPI placenta is unclear.
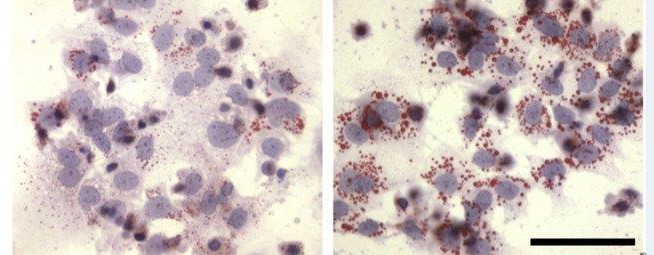
Figure 3: The images show oil red o-stained sections from male and female control and UPI placenta. Males are on the top and female are on the bottom. Increased red staining in the UPI male shows a greater lipid droplet accumulation adapted from https://doi.org/10.1210/jc.2004-2265.
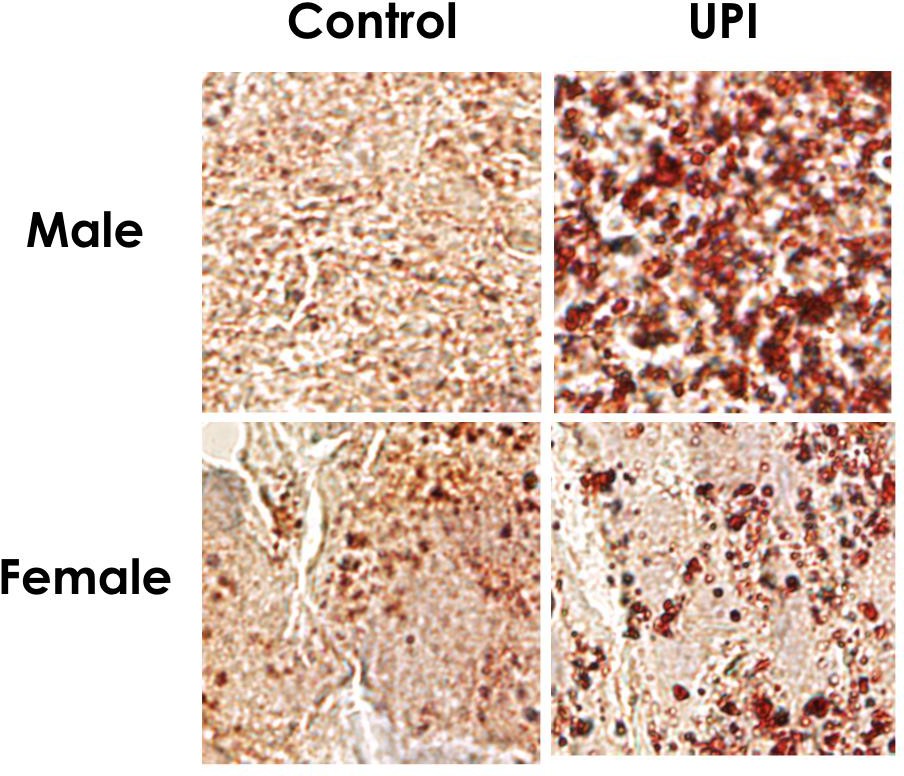
Figure 4: Male and Female Control and UPI Placenta Lipid Droplet Accumulation 4
The small red dots are the lipid droplets which are significantly increased in hypoxic compared to standard conditions. As shown, male UPI placenta has significantly worse lipid accumulation. In this study we aimed to determine what lipid classes with DHA and ARA fatty acids are most readily trapped in the placenta in UPI. We hypothesized that, in the rat, placental lipid accumulation in UPI will favor increased trapping of neutral lipids containing DHA and ARA.
Methods
All animal procedures were reviewed and approved by the University of Utah Animal Care and Use Committee, following the NIH Guidelines for the Care and Use of Laboratory Animals. UPI was induced in Sprague Dawley rats on embryonic day 19 via bilateral uterine artery ligation. The feto- placental units were then collected by c-section at term gestation or embryonic day 21. Untargeted lipidomics was used to determine lipid profiles in control and UPI placenta. For bulk analyses, MetaboAnalyst (https://www.metaboanalyst.ca/) was used to determine differential lipid levels, and Lipid Ontology enrichment analysis using LION software (https://pubmed.ncbi.nlm.nih.gov/31141612/) was used to determine differential lipid metabolism pathways. For granular analyses, Graphpad Prism was used to determine differences in placental levels of specific lipid species.
Results
For the bulk lipidomics data, there were no major differences between male and female placenta, so data is shown with the sexes combined. First, we used Metaboloanalyst to identify differential levels of lipid species in control and UPI placentas. There were 923 unique lipid species identified in the rat placenta, with 60 significantly increased and 43 significantly decreased in control and UPI placenta. Because we are interested in lipid classes increased in UPI, we focused on the increased species. Of these species over 90% were triglycerides (Figure 5).
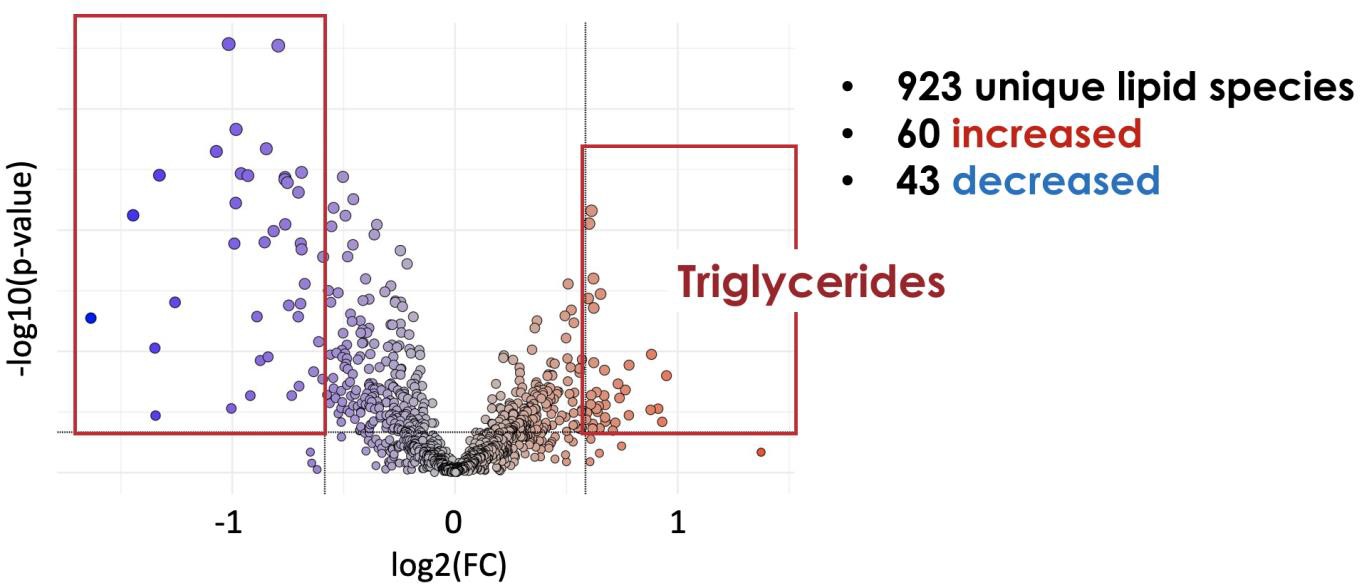
Figure 5: Volcano plot of lipid species in rat placenta
To understand the metabolic processes associated with the differences in the bulk lipidomic results, we also performed an enrichment analysis using the LION software. Given the results from the bulk lipidomic analysis showing increased lipid droplet formation primarily through increased triglyceride, we also analyzed all the triglyceride species at a more granular level. UPI increased total triglycerides in both male and female placenta. We also examined triglyceride species containing at least one DHA fatty acid chain. UPI increased placental DHA containing triglycerides in male, but not female, placenta (Figure 6). While for triglycerides with at least one ARA chain, UPI increased levels in both male and female placenta. Cholesterol esters were the most abundant neutral lipid in the placenta, so we also looked at the effect of UPI on cholesterol esters. UPI did not affect total cholesterol esters in either male or female placenta. UPI decreased cholesterol esters containing DHA in female placenta without affecting male placenta. UPI increased cholesterol esters containing ARA in the male placenta without affecting female placenta.
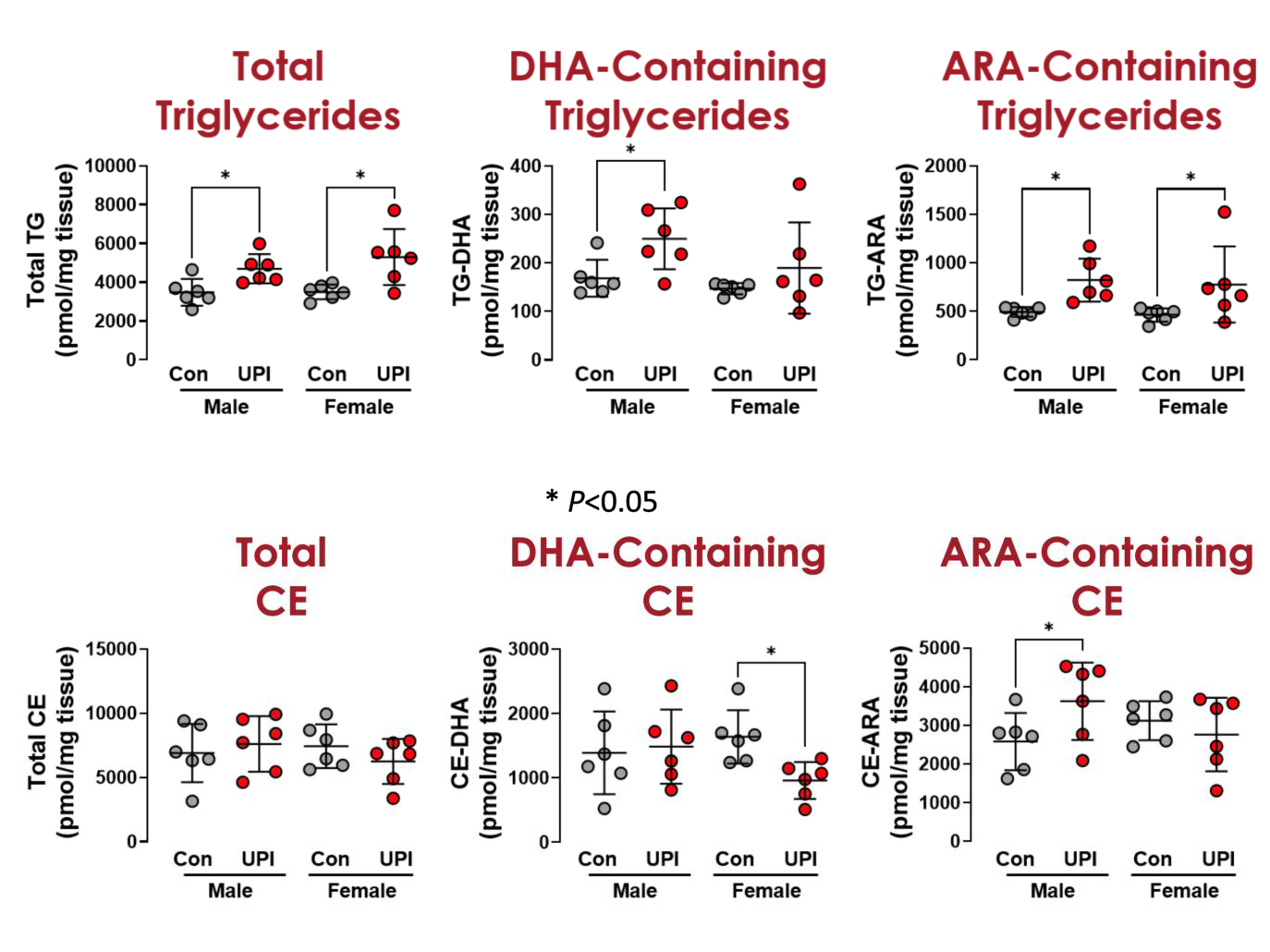
Figure 6: Individual circles are individual placentas from different litters. Males are on the left, females on the right. Control placenta shown as gray circles and UPI placenta shown as red circles, with bars as mean and standard deviation.
Conclusion and speculation
In conclusion, increased placental lipid accumulation in UPI placentas is characterized by an increase in neutral lipids, including those containing DHA and ARA fatty acids. We speculate that the accumulation of neutral lipids containing DHA and ARA in the UPI placenta prevents fetal acquisition of these bioactive fatty acids. We further speculate that the lipid droplet accumulation is driven by the increased PPARγ activity in UPI placenta and ongoing work is determining the cause-and-effect relationship between PPARγ activity and the lipid accumulation profiles shown previously.
References
- Jarc, E., & Petan, T. (2019). Lipid Droplets and the Management of Cellular Stress. The Yale journal of biology and medicine, 92(3), 435–452.
- W. Timothy Schaiff, Ibrahim Bildirici, Monica Cheong, Peggy L. Chern, D. Michael Nelson, Yoel Sadovsky, Peroxisome Proliferator-Activated Receptor-γ and Retinoid X Receptor Signaling Regulate Fatty Acid Uptake by Primary Human Placental Trophoblasts, The Journal of Clinical Endocrinology & Metabolism, Volume 90, Issue 7, 1 July 2005, Pages 4267–4275, https://doi.org/10.1210/jc.2004-2265
- Bildirici, I., Schaiff, W. T., Chen, B., Morizane, M., Oh, S. Y., O’Brien, M., Sonnenberg-Hirche, C., Chu, T., Barak, Y., Nelson, D. M., & Sadovsky, Y. (2018). PLIN2 Is Essential for Trophoblastic Lipid Droplet Accumulation and Cell Survival During Hypoxia. Endocrinology, 159(12), 3937–3949. https://doi.org/10.1210/en.2018-00752
- Stephanie Skuby Chassen, Veronique Ferchaud-Roucher, Madhulika B. Gupta, Thomas Jansson, Theresa L. Powell; Alterations in placental long chain polyunsaturated fatty acid metabolism in human intrauterine growth restriction. Clin Sci (Lond) 15 March 2018; 132 (5): 595–607. doi: https://doi.org/10.1042/CS20171340
- Barrett, E., Loverin, A., Wang, H., Carlson, M., Larsen, T. D., Almeida, M. M., Whitman, J., Baack, M. L., & Joss-Moore, L. A. (2021). Uteroplacental Insufficiency with Hypoxia Upregulates Placental PPARγ-KMT5A Axis in the Rat. Reproductive sciences (Thousand Oaks, Calif.), 28(5), 1476–1488. https://doi.org/10.1007/s43032-020-00434-w

