21 Identifying Drugs to Target Dysregulated Lipid Metabolism in Hepatocellular Carcinoma in Zebrafish
James Harris
Faculty Mentor: Kimberley Evason (Pathology, University of Utah)
Abstract
Previous work has been done to try and understand the molecular pathways at play in primary liver cancer. Lipid metabolism has been documented as being dysregulated in hepatocellular carcinoma (HCC). It is hypothesized that the pathway activated by the CTNNB1 gene plays an important role in the development of HCC and that this pathway could be used as a therapeutic target. The purpose of this paper is to examine the potential therapeutic use of 5 molecules to target this pathway: bezafibrate, meclofenoxate, sphinganine, choline and CDP choline. In these experiments a transgenic zebrafish was used to model HCC in humans. In this model the zebrafish are found to have enlarged livers at 6 days post fertilization and fully developed HCC as adults. Zebrafish were treated with the potential therapeutics from 3 to 6 days post fertilization and their liver sizes were measured as a marker for HCC. Bezafibrate, meclofenoxate, and sphinganine were found to not have any effect on liver size. While at specific dosage levels choline and CDP choline were seen to have an effect. Future work is necessary to determine if these molecules could be used as a therapeutic in humans, but the work done here provides evidence that the altered lipid metabolism pathways play an important role in the development of HCC-like changes in zebrafish.
Index Terms—Wnt pathway, b-catenin, ceramides, phosphatidylcholine
Introduction
Liver cancer represents a significant health burden worldwide. In 2020 alone 905,700 new patients were diagnosed with, and approximately 830,000 people died from liver cancer [1]. Of those diagnosed with liver cancer around 80% develop what is known as hepatocellular carcinoma (HCC), the most common type of primary liver cancer [2]. Understanding the different factors that lead to tumorigenesis in cancer patients is crucial to both treating and preventing cancer. One of the differences between normal healthy cells and cancerous HCC cells is the dysregulation of lipid metabolism.
One potential gene driver of liver cancer is a mutation in the CTNNB1 gene that codes for the protein b-catenin. This mutation is found in more than 20% of patients with HCC [3]. Mutations in the b-catenin gene lead to changes in the lipid metabolism pathway associated with it. In particular, the lipid species phosphatidylcholine (PC) and ceramide lipids have been seen to be dysregulated in HCC [4][5]. These changes could be responsible for the development of tumors in the liver. While the dysregulation of lipid metabolism in HCC is well documented, it is not yet fully understood what role lipid metabolism plays in tumorigenesis. To characterize which lipid synthesis and metabolism pathways are important in the dysregulated growth state of HCC, we examined what occurred when those pathways were inhibited using the drugs bezafibrate, meclofenoxate, sphinganine, choline and CDP choline in an activated b-catenin transgenic zebrafish line. Better understanding the role of the lipid metabolism pathway controlled by b-catenin could lead to the development of new therapeutic treatments. A better understanding of the cellular pathways that lead to tumorigenesis in the liver could improve patient outcomes.
Background
A. HepatocellularCarcinoma
Cancer is characterized by unregulated cell proliferation. In HCC, as in other kinds of cancer, the normal mechanisms to limit the proliferation of cells are damaged or otherwise altered allowing cells to replicate indefinitely. In serious cases, these rapidly growing cells can begin to migrate and invade other tissues in the body, a condition known as metastasis. Rapidly dividing cells have a much greater metabolic and nutritional need than normal cells. These needs lead to angiogenesis, or the formation of new blood vessels, as well as altered metabolic pathways. Altering lipid metabolism is one of the ways that cancer cells, particularly those found in the liver in HCC, meet the increased metabolic needs associated with rapid cell division.
B. Gene Expression
For HCC to develop, the cell must be able to meet a higher metabolic need and be supplied with the raw materials and nutrients necessary for continued cell proliferation. HCC driven by beta-catenin is commonly reported to exhibit dysregulated lipid metabolism [4], [5], [6] which could provide for both of those needs. PC and ceramide lipids in particular are seen to be dysregulated in HCC.
CTNNB1 mutations are observed in 20-40% of HCC patients on average [3]. CTNNB1 codes for the beta-catenin protein involved in the Wnt pathway. When this gene becomes altered or damaged altered beta-catenin proteins are produced that in some cases avoid degradation but remain able to bind to normal receptors. Such mutations drive cell proliferation even when the Wnt pathway would be otherwise disengaged.
C. Signaling Pathway
The Wnt signaling pathway has been identified as helping to regulate the activation and transcription of genes that control cell processes important not only in embryonic development but also in cancer [7]. The protein beta-catenin plays a crucial role in the function of this signaling pathway. The Wnt pathway relies on its ability to degrade cytoplasmic beta-catenin protein to control gene expression [7]. In the pathway’s activated state, Wnt binds to the Frizzled (Fz) or LPR5/6 receptor on the cell membrane. This deactivates the beta-catenin destruction complex allowing beta-catenin accumulation in the cytoplasm and translocation into the nucleus [7]. Once there, it binds to the lymphoid enhancer factor/T-cell factor (LEF/TCF), which leads to gene transcription. In its inactive state the Wnt ligand remains unbound to its Fz/LPR receptor which means the beta-catenin destruction complex remains active. This complex breaks down beta-catenin in the cytoplasm preventing it from translocating into the nucleus. LEF/TCF remains unbound, and gene transcription does not occur. Mutations in the proteins involved in the destruction complex or in the beta-catenin itself can lead to the hyperactivation of beta-catenin and the over-expression of those genes involved in cell proliferation.
D. Zebrafish Model
Certain characteristics of zebrafish make them a useful animal model from an experimental perspective. For example, drugs can be administered more uniformly across multiple specimens because drugs can simply be dissolved in the water in which the fish are maintained. Additionally, in the early stages of development zebrafish larvae are transparent allowing for easy observation of internal processes relevant to HCC. Aside from these experimental advantages, zebrafish are also an effective animal model from a biologic perspective. About 86% of 1318 human drug targets and 70% of all human genes were found to have at least one zebrafish orthologue [8] [9]. Their genetic similarity to humans makes zebrafish useful in modeling many different diseases including HCC. When the CTNNB1 gene that controls b-catenin expression is turned on, zebrafish specimens develop HCC that morphologically and genetically resembles human HCC [10]. This is critically important when trying to understand the molecular processes that control this disease. Activated b-catenin (ABC) fish begin to show markers of HCC in early development allowing for relatively fast observation of the effects of certain biologic pathways on cancer development. Specifically, ABC fish exhibit enlarged liver sizes when compared to wild type (WT) fish at just 6 days post fertilization (dpf). This change in liver size can be used as a surrogate marker for studying HCC. These physical and genetic characteristics make a zebrafish animal model advantageous for studying human HCC.
E. Potential Therapeutics
Dysregulated PC and ceramide metabolism pathways are promising potential therapeutic targets for HCC treatment. To modulate the metabolism of PC and ceramides in the liver there are many molecules that could be used. Different molecules act on different parts of the lipid synthesis and metabolism processes in the cell. Bezafibrate inhibits Phosphatidylethanolamine N-methyltransferase (PEMT) [11] and meclofenoxate (also known as centrophenoxine) inhibits Choline Phosphotransferase (CPT) [12] which both contribute to the synthesis of PC. Choline and CDP-choline are both considered supplemental molecules that are naturally involved in the production of PC [12]. Sphinganine is another supplemental molecule and is a crucial part of the synthesis of ceramides [13].
METHODS
A. ObtainingLarvaeforTreatment
Embryos were obtained from established zebrafish lines expressing the activated b-catenin pathway in order to test the effect of the selected drugs on HCC development. WT and ABC zebrafish were crossed, and the collected embryos were allowed to develop for 3 days. The transgenic line used for this experiment expresses GFP in the eyes when the CTNNB1 gene is activated. At 3 days post fertilization larvae were sorted into groups based on gene expression by using the observable expression of GFP in the eyes as a marker for the activated b-catenin pathway. In the cross between WT and ABC zebrafish, the embryos come from the same parent fish and so are genetically comparable. It is expected that approximately half the larvae from a single cross will express the activated b-catenin pathway and enlarged larval liver size associated with HCC. The other half will exhibit a normal WT phenotype.
B. Experimental Treatment
Once larvae are divided into WT and ABC groups, they were placed in the drug treatment. Larvae were placed in groups of 20 in 10 mL well plates at specific testing concentrations. Meclofenoxate, bezafibrate, sphinganine, choline and CDP choline were all obtained in pure powder form and dissolved in DMSO or water at the highest concentration possible given their solubility. Meclofenoxate, bezafibrate and sphinganine were all dissolved in DMSO while choline and CDP choline were dissolved in water. Drug gradients for the treatments using meclofenoxate and bezafibrate were created by diluting the stock DMSO-drug solution to 100 mM, 50 mM, and 25 mM concentrations. 10 μL of each solution were added to the 10 mL wells housing the larvae groups creating a drug concentration within the well of 100 μM, 50 μM and 25 μM respectively. 10 μL of stock solution were added to one of the groups to create a positive control group used to determine if the drug was being absorbed by the larvae. Similarly, 10 μL of DMSO was added to one well used to create a negative control group used to determine the effect of DMSO on the larvae in the absence of any additional drug molecule. The meclofenoxate drug experiment had treatment groups of both WT and ABC fish treated with 100 μM, 50 μM, 25 μM, and a 0 μM negative control (NC) concentrations of meclofenoxate. Similarly, the bezafibrate experiment tested separate groups of both WT and ABC zebrafish treated at 100 μM, 50 μM, 25 μM, and 0 μM (negative control) concentrations of bezafibrate.
Sphinganine, choline and CDP choline were all obtained in powder form. The powder form of the drug was dissolved in solvent at the highest concentration possible and then diluted down to the desired treatment concentrations.
Like meclofenoxate and bezafibrate sphinganine was dissolved in DMSO and diluted to the desired treatment concentrations. WT and ABC fish were treated with 0 μM, 1 μM, 2.5 μM and 4 μM of sphinganine. Because sphinganine was dissolved in DMSO the negative control group was administered 10 μL of the DMSO solvent. Sphinganine is considered a supplementary molecule and so a positive control group was not used for this set of experiments.
Choline and CDP choline are also considered supplementary molecules and so did not require a positive control treatment group for experimentation. Experimental treatments with choline treated fish with 0 μM, 250 μM, 500 μM, 750 μM, and 1000 μM concentrations of the drug. CDP choline experiments treated fish with 0 μM, 10 μM, 25 μM, 50 μM and 102 μM concentrations of the drug. Unlike sphinganine which was dissolved in DMSO, choline and CDP choline were dissolved in water. No solvent addition was necessary for the negative control groups in these experiments.
C. Fixing and Imaging Larval Specimens
Larvae were left in the treatment wells and kept in an incubator for 3 days. At this point, larvae were placed on ice for 20 min to induce euthanasia. Euthanized zebrafish were placed in PFA for 24 hrs. to fix the specimens. After this fixation period the outer layer of skin was removed from each larva using forceps to allow for easy imaging of the liver. Larvae were then imaged using a Leica Confocal Microscope. Larvae were oriented such that when on their side their left and right eye were directly on top of one another. This placement ensured that each larval liver was observed from the same angle and that any difference in observed size was due to an actual size difference between livers and not just a difference of perspective.
D. AnalyzingLiverSize
Once images were taken of each larva the pictures were randomized to blind observers as to the test condition. The area of the livers was measured using ImageJ2 (version 2.14.0/1.54f). Pixel area measurements are then converted into mm2 areas using the established conversion scale. After sizes were obtained, the files were derandomized into their original treatment groups. Differences in the liver size between groups were determined based on the average liver size for the group, and significance was determined using a one-way analysis of variance (ANOVA) test.
RESULTS
A. Meclofenoxate
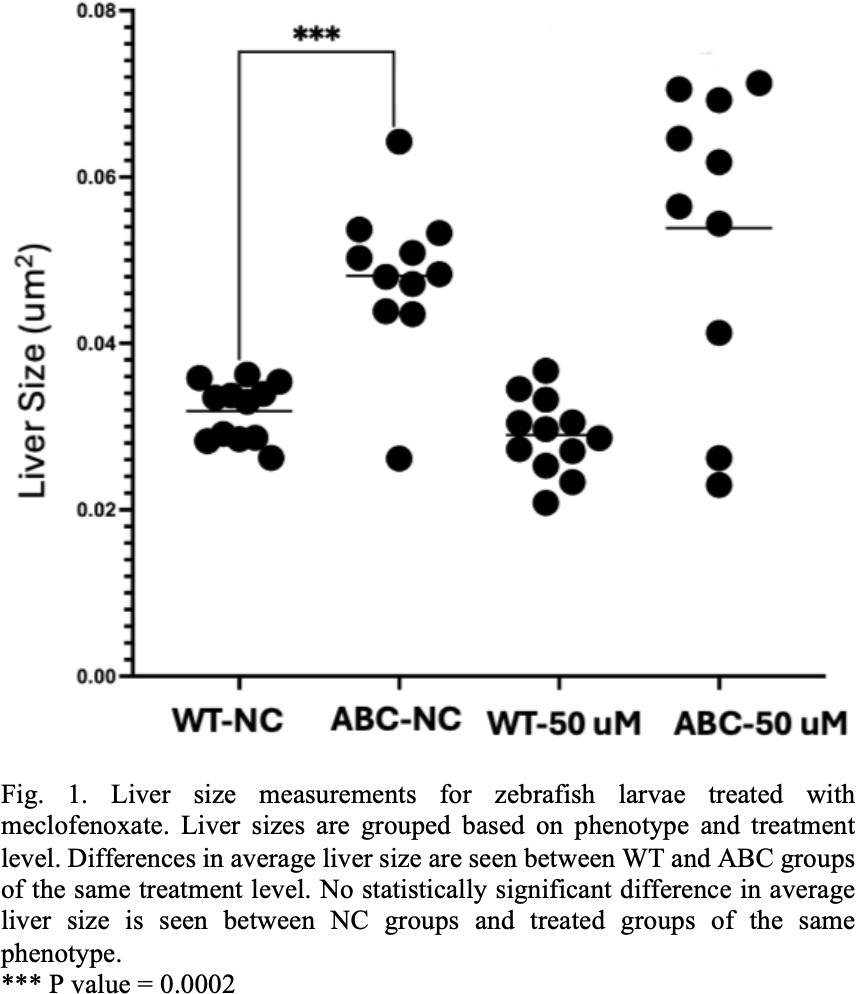
The WT groups showed no significant difference in liver size when treated with meclofenoxate as was expected. The zebrafish larvae in the 0 μM concentration negative control group showed an enlarged liver size when compared to their WT sibling controls. However, the ABC line continued to exhibit enlarged liver sizes when treated with 25 μM, 50 μM, and 100 μM concentration of meclofenoxate. The 50 μM treatment comparison was considered representative of the results from other test concentrations and can be seen in Figure 1.
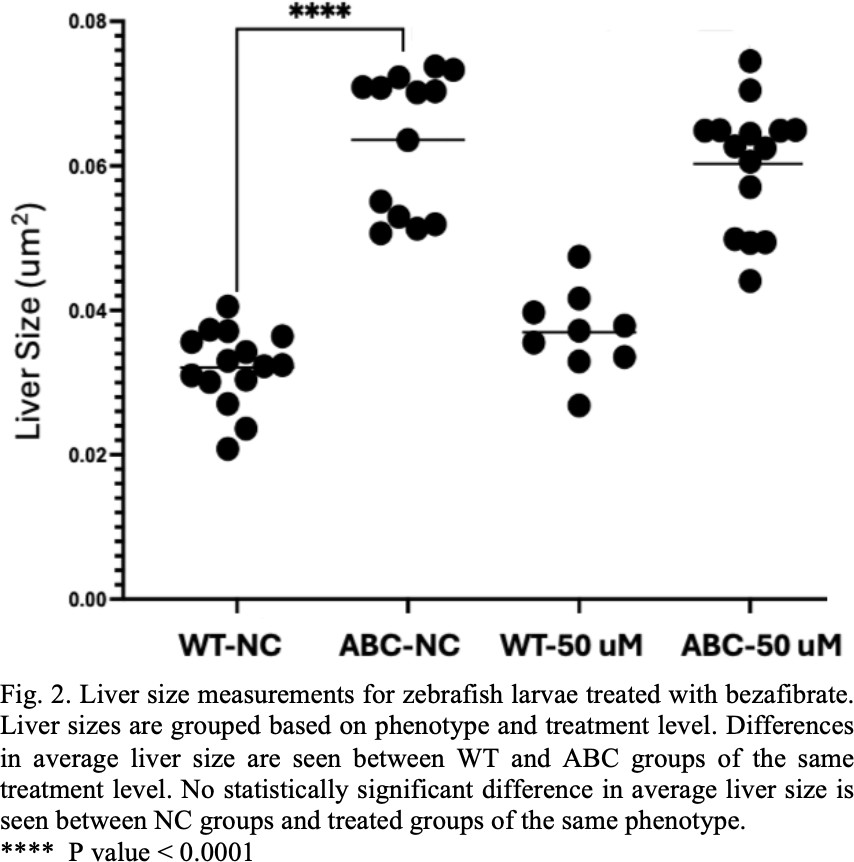
The WT groups showed no significant difference in liver size when treated with bezafibrate. The negative control, ABC group showed significant difference in liver size from the WT groups. The ABC line continued to exhibit enlarged liver sizes when treated with 25 μM, 50 μM, and 100 μM concentration of bezafibrate. The 50 μM treatment comparison was considered representative of the results from other test concentrations and can be seen in Figure 2.
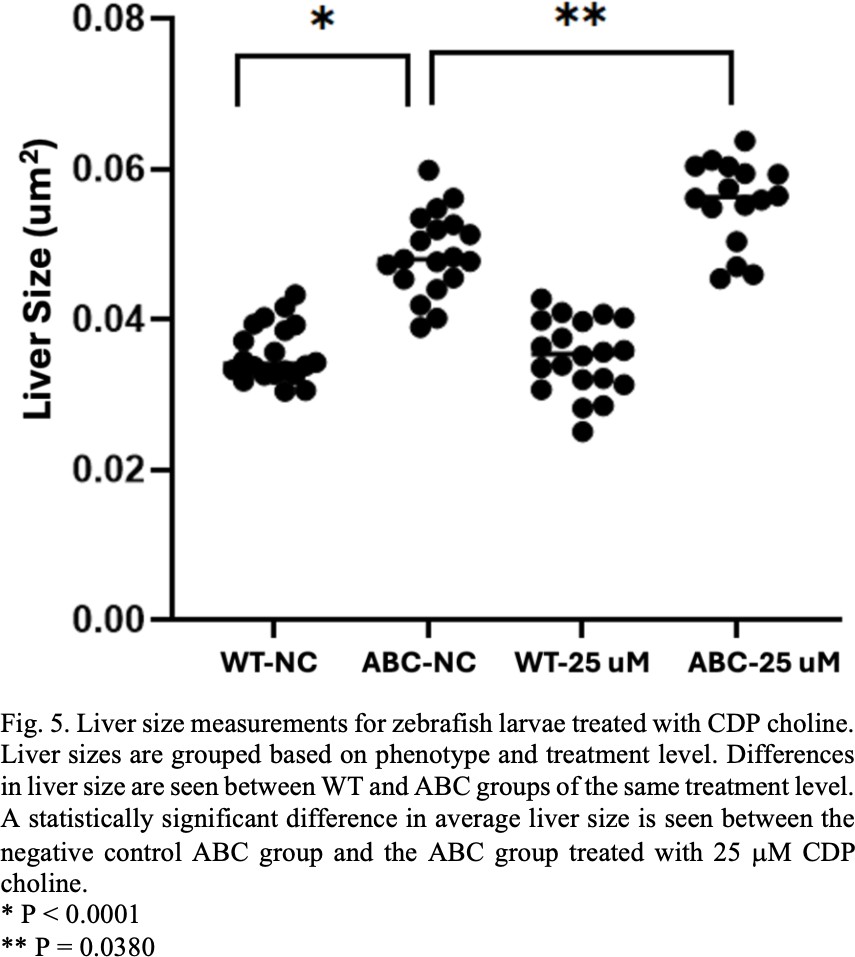
In the CDP choline experiments no significant difference in liver size was observed between the WT groups when treated with choline. A significant difference in average liver size was seen in the ABC larvae treated with 25 μM CDP choline in comparison to the negative control ABC group. No significant difference in liver size was seen between the ABC zebrafish groups treated with other dosage levels in comparison to the negative control ABC group. Comparison between the WT and ABC negative control groups as well as a comparison between the negative control group and the group treated with 25 μM CDP choline can be seen in Fig. 5.
One of the most common kinds of primary liver cancer, hepatocellular carcinoma (HCC), is known to exhibit dysregulated lipid metabolism. Notably the two lipid types, phosphatidylcholine (PC) and ceramides, are found in different levels in HCC cells than in healthy cells [4], [5], [6]. It has been hypothesized that the synthesis and metabolism pathways involving these molecules could be targets for novel cancer treatments.
In order to better understand these pathways, we examined the effect of five drugs: meclofenoxate, bezafibrate, sphinganine, choline and CDP choline that are known to play a role in the synthesis and metabolism pathways of PC and ceramides. We used an activated b-catenin (ABC) zebrafish line as a model for HCC in humans. Zebrafish larvae expressing the activated b-catenin protein exhibit enlarged livers when compared to their wild type (WT) siblings [3]. Enlarged larval liver size was used as a marker for HCC-like changes in the developing fish. Drugs were administered to the zebrafish larvae globally from 3 to 6 days post fertilization. After the treatment period the specimens were imaged so that their liver sizes could be measured.
As seen in Figure 1 and Figure 2. there was a significant difference in liver size between the WT and ABC control groups, but no significant difference was seen between the meclofenoxate or bezafibrate treated and untreated groups. The enlarged livers we observed are indicative of the expected changes taking place in the larvae in response to the activation of the b- catenin protein. The lack of observed change in liver size in response to the drug treatment could be explained by a number of factors.
In early experiments we saw significant amounts of technical issues in the imaging process. In some cases, larvae became damaged in preparation and in other cases larvae were misaligned and liver areas could not be measured accurately. It is possible that some of these early issues effected these experiments. Each treatment group started with 20 individuals. The fact that in some of the treatment groups within the meclofenoxate and bezafibrate experiments as little as 50% of the specimens were measured calls these results into question.
Other work done on the subject also contradicts the results seen here in the meclofenoxate and bezafibrate experiments. For example, the work done by Evason et al [3] showed that meclofenoxate and bezafibrate reduced liver size when administered to ABC zebrafish larvae. These experiments failed to replicate the same results, but it is suspected that this was caused by experimental error. Future experiments should try and eliminate error so a stronger comparison could be made to the other work that has been done.
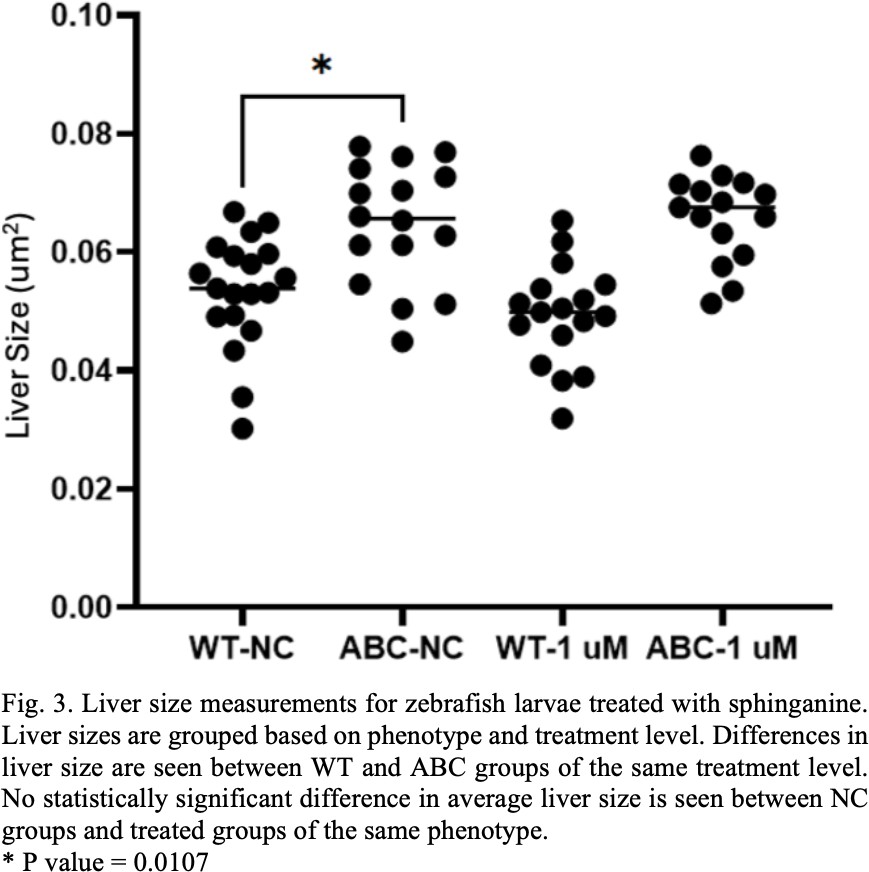
One of the biggest differences between the work done elsewhere in literature and the work done here is the incorporation of sphinganine, choline and CDP choline. In the sphinganine treatment experiment a significant difference was seen in the larval liver size between the WT and ABC negative control groups, as was expected. However, no significant difference was observed between negative control and sphinganine treated groups (see Fig. 3). While there was not an observable difference in liver size in response to drug treatment, these experiments did identify a useful range of sphinganine concentrations that zebrafish larvae could tolerate. The larvae did not tolerate the 2.5 M and 4 M concentrations but could tolerate the 1 μM concentration they were treated with. Future work should examine other concentrations around 1 μM that could be tolerated and might still have an effect on liver size. If other experiments yield similar results, it could mean that the ceramide synthesis and metabolism pathway that sphinganine is a part of does not play a significant role in HCC development.
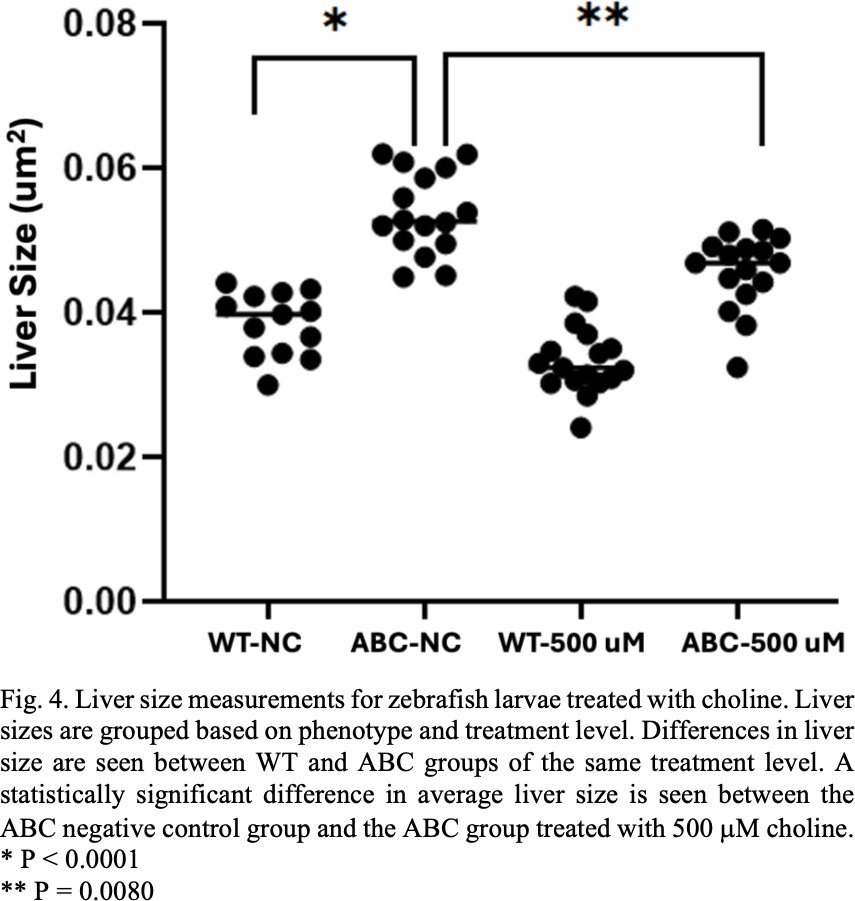
Unlike the meclofenoxate, bezafibrate and sphinganine treatments a difference in liver size was observed when the ABC larvae were treated with choline and CDP choline. When treated with a 500 μM concentration of choline the ABC zebrafish showed significant differences in liver size when compared to the ABC negative control groups (see Fig. 4). In the PC synthesis pathway choline is modified by a series of molecular processes and is eventually used in creating more PC for the cell [12]. Treated larvae had an average liver size that was smaller than the untreated group. This could mean that the addition of choline into the system is somehow contributing to other processes that are reducing cell proliferation. Further study is necessary to determine what exactly is going on.
In the reaction cascade that eventually converts choline to PC one of the choline products is converted into CDP choline [12]. Since both molecules are part of the same chain that leads to PC synthesis, it could be hypothesized that adding either molecule into the system would have similar effects. This was not the case in our observations. The group of ABC fish that was treated with 25 μM of CDP choline saw a significant difference in average liver size when compared to the ABC untreated group (see Fig. 5). This group had an average liver size that was larger than the negative control group. This could mean that CDP choline and the enzymes that convert it into PC play a significant role in HCC development. Inhibiting choline conversion into CDP choline or CDP choline conversion into PC could be successful in controlling the rapid cell proliferation necessary for HCC to develop.
Treatment and control groups were limited in size and so were more affected by variation between individuals. The data collected could be made more robust by testing a larger group. Additionally, the drug doses tested here represent only a fraction of the potential dose concentrations. While the treatment doses were chosen thoughtfully and intentionally, there remains opportunity for further testing using other drug concentrations. It is possible that the lack of therapeutic effect observed from some of the drugs was due to an insufficient dose.
While these experiments contribute to our understanding of how dysregulated lipid metabolism effects the development of HCC, they also highlight gaps in our understanding. Without further testing we can’t know for certain if the differences between the results observed in these experiments with meclofenoxate and bezafibrate and the results of similar experiments were in fact due to experimental error. We do not yet know why choline and CDP choline had the effects that they did, or why sphinganine did not have a visible effect. Repeating similar experiments while eliminating the known sources of error could determine whether future mechanistic studies merit further pursuit.
The dysregulated lipid metabolism pathways seen in HCC cells continue to show promise as a therapeutic target. The work described in this paper helps to pave the way for future work that could change the face of liver cancer treatment.
References
[1] Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022 Dec;77(6):1598-1606. doi: 10.1016/j.jhep.2022.08.021. Epub 2022 Oct 5. PMID: 36208844; PMCID: PMC9670241.
[2] American Cancer Society, “Cancer Facts & Figures – 2012,” 2012.
[3] K. J. Evason, Francisc, M.T., Juric, V., Balakrishna, S., Pazmino, MPL, Gordan, J.D., Kakar, S., Spitsbergen, J., Goga, A., & Stainier, D.Y.R. “Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish,” PLOS Genetics, vol. 11, no. 7, p. e1005305, Jul. 2015, doi: 10.1371/journal.pgen.1005305.
[4] VanSant-Webb C, Low HK, Kuramoto J, Stanley CE, Qiang H, Su A, Ross AN, Cooper CG, Cox JE, Summers SA, Evason KJ, Ducker GS. Phospholipid isotope tracing reveals β-catenin-driven suppression of phosphatidylcholine metabolism in hepatocellular carcinoma. bioRxiv [Preprint]. 2023 Oct 16:2023.10.12.562134. doi: 10.1101/2023.10.12.562134. Update in: Biochim Biophys Acta Mol Cell Biol Lipids. 2024 Aug;1869(6):159514. doi: 10.1016/j.bbalip.2024.159514. PMID: 37904922; PMCID: PMC10614757.
[5] Krautbauer S, Meier EM, Rein-Fischboeck L, Pohl R, Weiss TS, Sigruener A, Aslanidis C, Liebisch G, Buechler C. Ceramide and polyunsaturated phospholipids are strongly reduced in human hepatocellular carcinoma. Biochim Biophys Acta. 2016 Nov;1861(11):1767-1774. doi: 10.1016/j.bbalip.2016.08.014. Epub 2016 Aug 26. PMID: 27570113.
[6] I. T. Ismail, A. Elfert, M. Helal, I. Salama, H. El-Said, & O. Fiehn, “Remodeling Lipids in the Transition from Chronic Liver Disease to Hepatocellular Carcinoma,” Cancers (Basel), vol. 13, no. 1, p. 88, Dec. 2020, doi: 10.3390/cancers13010088.[7] J. Liu et al., “Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities,” Sig Transduct Target Ther, vol. 7, no. 1, pp. 1–23, Jan. 2022, doi: 10.1038/s41392-021-00762-6.
[8] L. Gunnarsson, A. Jauhiainen, E. Kristiansson, O. Nerman, & D. G. J. Larsson, “Evolutionary Conservation of Human Drug Targets in Organisms used for Environmental Risk Assessments,” Environ. Sci. Technol., vol. 42, no. 15, pp. 5807–5813, Aug. 2008, doi: 10.1021/es8005173.
[9] Howe, K., Clark, M., Torroja, C. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013). https://doi.org/10.1038/nature12111
[10] J. Nakayama and Z. Gong, “Transgenic zebrafish for modeling hepatocellular carcinoma,” MedComm, vol. 1, no. 2, p. 140, Sep. 2020, doi: 10.1002/mco2.29.
[11] Tomoko Nishimaki-Mogami, Kazuhiro Suzuki, Eriko Okochi, & Atsushi Takahashi, “Bezafibrate and clofibric acid are novel inhibitors of phosphatidylcholine synthesis via the methylation of phosphatidylethanolamine,” Biochimica et Biophysica Acta (BBA) – Lipids and Lipid Metabolism, vol. 1304, no. 1, pp. 11–20, Nov. 1996, doi: 10.1016/S0005-2760(96)00101-4.
[12] A. K. Percy, J. F. Moore, & C. J. Waechter, “Phosphoglyceride biosynthesis by brain microsomes: centrophenoxine, SaH-42-348, and DH-990 inhibit phospholipid N-methylation,” Arch Biochem Biophys, vol. 235, no. 1, pp. 18–25, Nov. 1984, doi: 10.1016/0003-9861(84)90250-9.
[13] C. Sohlenkamp, I. M. López-Lara, & O. Geiger, “Biosynthesis of phosphatidylcholine inbacteria,” Progress in Lipid Research, vol. 42, no. 2, pp. 115–162, Mar. 2003, doi: 10.1016/S0163-7827(02)00050-4.