John and Marcia Price College of Engineering
19 Quantification and Characterization of Microplastics in Biosolids and Soils Across the Wasatch Front
Aspen Dalby and Jennifer Weidhaas
Faculty Mentor: Jennifer Weidhaas (Civil and Environmental Engineering, University of Utah)
ABSTRACT
Microplastics (MPs) are present in biosolids and soils where biosolids are applied, raising concerns about their potential accumulation and impact on soil health. This study quantifies microplastic levels in biosolids and agricultural soils in the Wasatch Front Region to better understand their abundance, types, and implications. MPs were isolated using chemical and physical separation techniques, visually identified through microscopy, and further analyzed for chemical composition using Fourier Transform Infrared Spectroscopy (FTIR), with a self-generated reference library and external literature for verification.
Results show that biosolids contained 32 to 43 MPs per gram, while biosolid-amended soils had 20 to 28 MPs per gram. In comparison, control soils, where biosolids were not applied, contained 8 MPs per gram, suggesting previous presence of MPs. Based on these findings, it is estimated that 9.4 × 10⁸ MPs could be introduced into agricultural soils over a five-year period through biosolid application based on a biosolids application report for a treatment plant in the Wasatch Front [1]. MPs affect plant growth, enzyme activity, and soil health by altering microbial diversity, soil structure, and nutrient cycles [2]. Plants can uptake MPs through root absorption, leaf adsorption, or water uptake, potentially leading to bioaccumulation in edible crops and posing risks to human health [3]. This underscores the need for ongoing monitoring and research of MPs in biosolids and soils to guide sustainable land management practices in the Wasatch Front region.
1. INTRODUCTION
Plastic waste remains a significant global concern and is expected to continue increasing. Projections indicate that plastic waste could nearly double between 2020 and 2050, even with improved recycling initiatives [4]. Plastic pollution in the natural and built environment is alarming, and it is important to consider the extent to which this poses a risk for humans and ecosystems.
Plastic waste can degrade into small pieces through biological processes or abiotic factors like ultraviolet radiation or mechanical stress [5]. This degradation creates a category of particles called microplastics (MPs). MPs range in size from roughly 1 to <1000 micrometers that can break into smaller categories like nanoplastics (1 to <1000 nanometers) [6], and are sourced from various entities such as single-use plastics, manufactured products, household items, and automotives [7]. Imagine your daily routine and think about how many times you encounter plastic products from the moment you wake up to when you go to sleep. They are arguably an integral part of our world. MPs can be further set apart by physical, chemical, and mechanical properties, which all play a role in MPs’ environmental fate and transport [8].
Similar to plastics, wastewater originates from multiple sources, including domestic activities (e.g., toilet flushing), industrial processes (e.g., manufacturing), and runoff, all of which contribute to wastewater entering treatment plants. Wastewater contains elements like microorganisms, biodegradable organic materials, metals and inorganic materials [9]. Additionally, MPs can enter the wastewater system. For example, polyethylene microbeads, commonly found in hygiene products, are estimated to release approximately 260 tonnes of microbeads annually in the United States (2.6 × 10⁸ grams) and 450 tonnes per year in the European Union (4.5 × 10⁸ grams). The actual quantities may be higher, as some MPs are retained during various stages of the wastewater treatment process [10]. It is shown that MP retention during treatment can be high, with secondary treatment systems retaining between 83.0% and 98.4% of MPs, and primary and tertiary treatments achieving up to 99.9% retention in some cases [10]. Most MPs end up in the solid byproducts of wastewater treatment, with 2% exiting via the effluent. About 4% are detected in the sludge, and the remaining 94% may be present in the sludge but go undetected due to limitations in current analytical methods [11].
The Environmental Protection Agency (EPA) looks at a term called contaminates of emerging concern (CECs) to describe an environmental pollutant that is not currently routinely monitored but has reason to be considered for routine monitoring and may pose a concern to the health of humans and the environment [12]. MPs fit into this category, which emphasizes the importance of understanding and monitoring their different pathways. Being able to categorize and track MPs is key for assessing public health implications. While methods for characterizing and quantifying MPs vary, identifying where they exist and how they move remains essential.
An area of the natural environment that MPs can enter is soils. Agricultural soils are especially at risk of microplastic contamination because crops require nutrients for growth, often provided by natural fertilizers like sewage sludge, called biosolids. Across the Wasatch Front region in Utah, hundreds of tons of biosolids are applied per year according to a local report [1]. They are delivered to a biosolids staging area (Figure 1) and then spread across pastures as needed (Figure 2).

Figure 1. Biosolids staging area

Figure 2.. Biosolids applied to an agricultural field
Biosolids are known to have a concentration of MPs as a byproduct of the wastewater treatment process that cannot completely eliminate MPs yet [13]. This raises the question about how microplastic concentrations vary in biosolids and the extent to which they impact soil health. To explore the relationship between MPs and soil health, one study exposed soils growing rye grass to three different types of MPs. One key finding showed a 7 percent reduction in seed germination compared to a control when soils growing rye grass were exposed to microplastic clothing fibers [14]. Though this study is limited in the types of MPs and soil environments analyzed, it highlights precisely why it is crucial to quantify and understand the concentration of MPs in soils and agricultural products like biosolids.
Limitations in quantifying MPs arise due to the variability in plastic types from sample to sample. As a result, physical and chemical techniques are often combined when identifying MPs. Physical methods include microscopy for visually quantifying MPs. Chemical methods can be destructive or non- destructive to a sample and can reveal details about the chemical structure of MPs. Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) are two non-destructive chemical techniques that use light and the electromagnetic spectrum to reveal material properties [15]. The choice between these methods often depends on particle size and the balance between time and labor.
The objective of this study is to better understand the mass of MPs present in biosolids from wastewater treatment plants and in agricultural fields across the Wasatch Front Region, where biosolids have been historically applied. Given the region’s use of biosolids in agriculture, this research aims to assess the amount and types of MPs, offering valuable insights for future studies on their potential impact on soil health.
Previous studies have demonstrated that MPs are consistently present at various stages of the wastewater treatment process with biosolids containing 5 MPs per 5g of biosolids in Southern California [16]. A case study in the state of Washington highlighted the accumulation of MPs in soils, particularly in biosolid-amended plots, where soil samples showed mean concentrations in the range of a few hundred MPs per kg (a few MPs per g), with the highest number observed in medium biosolid-amended soils (544 MPs/kg or 0.544 MPs/g) [17]. However, data on MPs in biosolids and soils along the Wasatch Front region, with its unique soil composition and environmental conditions, remain limited. This study investigates MP presence in regional biosolids and soils to aid understanding of long-term effects of MPs on soils and soil health. The findings will support sustainable biosolid management and inform policy decisions to mitigate microplastic contamination.
2. METHODS
2.1 Microplastic Extraction and Digestion from biosolids, soils, and plant roots
For a more efficient extraction of MPs from solids like biosolids and soils, it is recommended that organic matter (e.g., microbial cells) is separated via chemical digestion and physical separation using density gradient floatation [18]. For example, coupling digestion by 30% Hydrogen Peroxide (H2O2) at 70°C with a high-density salt solution of Sodium Iodide (NaI) is an optimal combination for extracting small and high density MPs [18].
In this study, methods were adapted from Ruffell et al. [19] for the extraction of MPs from solid biowastes. The protocol employed Fenton’s Reagent, prepared by combining 50 mL of iron (II) sulfate, 50 mL of 30% hydrogen peroxide (H₂O₂), and 50 mL of deionized water. Ultrapure water, as specified in the original method, was replaced with deionized water due to availability constraints.
Each trial involved the treatment of approximately 30 g of biosolids or soil. Soils were additionally pretreated by sieving through a #10 sieve prior to digestion. To sustain the reaction, an additional 20 mL of reagent was introduced twice daily throughout the digestion period as described in the method.
Complete digestion was achieved in approximately seven days for biosolid samples (Figure 3) and in approximately three to four days for agricultural soil samples, based on visual observations. As outlined in the original method, digestion was considered complete when organic matter had settled, turbidity was low, and no further bubbling was observed. At this stage, ~200 mL of deionized water was added to facilitate the degradation of residual H₂O₂.
A control experiment was incorporated into the digestion and extraction process to provide a comparative reference for method performance. Known quantities of Polypropylene (PP) and Polyethylene (PE) at 0.1 g each were spiked into the control sample, which was processed using the same digestion and density gradient separation procedures applied to biosolid and soil samples. This allowed for an evaluation of the recovery efficiency of the extraction method.
Additionally, a soil sample from a control field, which had not been amended with biosolids, was subjected to the same digestion and density gradient separation procedures to examine potential pre-existing microplastic contamination in the soil. Microscopy counts were performed to assess the presence of MPs in this control soil sample.

Figure 3. Fenton’s digestion on biosolids over seven days
2.2 Density Gradient Separation
Following digestion, the supernatant underwent first, a density separation using deionized water. In contrast to the original Ruffell method, which employs a Sediment-Microplastic Isolation (SMI) unit, the initial density separation was conducted in a 1L beaker. The resulting supernatant was divided into two parts: one was subjected to vacuum filtration using a Mixed Ester Cellulose 0.45 µm filter (Figure 4), while the other was resuspended in a salt solution for further processing (Figure 5).

Figure 4 .Vacuum Filtration Setup
The original method specifies the use of sodium iodide (NaI) at a density of 1.8 g/cm³ for density separation. In this study, sodium chloride (NaCl) at a concentration of 358 g/L (1.19 g/cm³) was selected instead. Among various tested solutions— NaCl (358 g/L, 1.19 g/cm³), NaBr (905 g/L, 1.53 g/cm³), and sodium hexametaphosphate (SHMP, added until saturation, yielding 1.30 g/cm³)— NaBr yielded the highest recovery rates for conventional plastics. However, NaCl demonstrated sufficient efficacy in recovering PP and PE [20], aligning with the control materials used in this study.

Figure 5. Density Separation using 358g/L NaCl
Following the second density separation, the supernatant was subjected to vacuum filtration, yielding a second filter. Additionally, the settled solids from the initial digestion were resuspended in 100 mL of 358 g/L, to assess potential microplastic recovery, representing a deviation from the original method. This step generated a third filter, and all three filters were then analyzed.
2.3 Control and QA/QC
A control experiment was conducted alongside biosolids and biosolid-amended soils, using the same reagents, and spiked with known quantities of MPs, as described in the original method (Figure 6). A review of 65 studies on MPs in biosolids identified PP, PE, and polyamide/nylon as the most commonly detected polymers [21]. While the original method recommends spiking the control with 1× reference fragments (500–1000 µm) of PET, PVC, HIPS, PP, ABS, HDPE, and PA, along with 10× PE microbeads and 10× PMMA fibers, the present study utilized 0.10 g of PP and 0.10 g of PE (32 mesh), sourced from Magerial Science. These polymers were selected based on their prevalence in the reviewed literature and their availability, with the full range of plastics not being feasible to source.
To assess the presence of pre-existing microplastic contamination in soils receiving biosolids, a control field not amended with biosolids was included in the study. This control field underwent the same digestion and density gradient separation procedures as the biosolid-amended samples to evaluate any potential microplastic contamination before the application of biosolids.

Figure 6. Control spiked with equal parts 32 mesh PP and PE
Quality assurance and control were maintained by minimizing the use of plastic laboratory equipment, opting for glassware to reduce the risk of plastic contamination. Gloves were changed as needed, and work surfaces were thoroughly cleaned to prevent contamination. Beakers were properly cleaned before each trial using a diluted Micro-90 solution to reduce dust contamination. Special attention was given when handling filters, ensuring they were directly transferred from the sterile covering into the filtration device via clean tweezers to prevent any potential contamination during the filtration process.
Microscopy counts were carried out using an AmScope SM-1 Series Zoom Binocular Stereo Microscope (7X–45X magnification) equipped with a 5 MP USB 2.0 C-mount camera on a track stand. At 45X magnification, the filter was divided into two sections, and half was analyzed for microplastic content. Particles exhibiting irregular shapes and diverse colors, as well as any material with characteristics indicative of plastic, were documented and counted. The observed counts were then doubled to estimate the total count for the entire filter. These values were subsequently adjusted based on the recovery efficiency determined from the control sample (see Appendix I).
2.5 FTIR-ATR analysis
For biosolid samples, select suspected microplastic particles were further analyzed using Fourier Transform Infrared Spectroscopy (FTIR) with a Nicolet iS50 FT-IR spectrometer fitted with a single-bounce Attenuated Total Reflectance (ATR) crystal. ATR-FTIR spectroscopy was chosen due to its strong correlation coefficients (r > 0.90) across multiple polymer types, providing reliable identification of MPs in biosolids [22].
Initially, the entire filter containing the suspected plastics was scanned, and a manual subtraction of the filter background was attempted. However, this approach resulted in unreadable spectra. Consequently, an alternative method was employed: suspected microplastic particles, barely visible to the naked eye, were carefully extracted from the filter using tweezers and placed directly on the ATR crystal for scanning. This approach presented challenges, including potential contamination from ambient dust and difficulty isolating plastic particles without unintentionally collecting adhered materials. As a result, while the obtained spectra were more interpretable, contamination remains a source of error in the analysis.
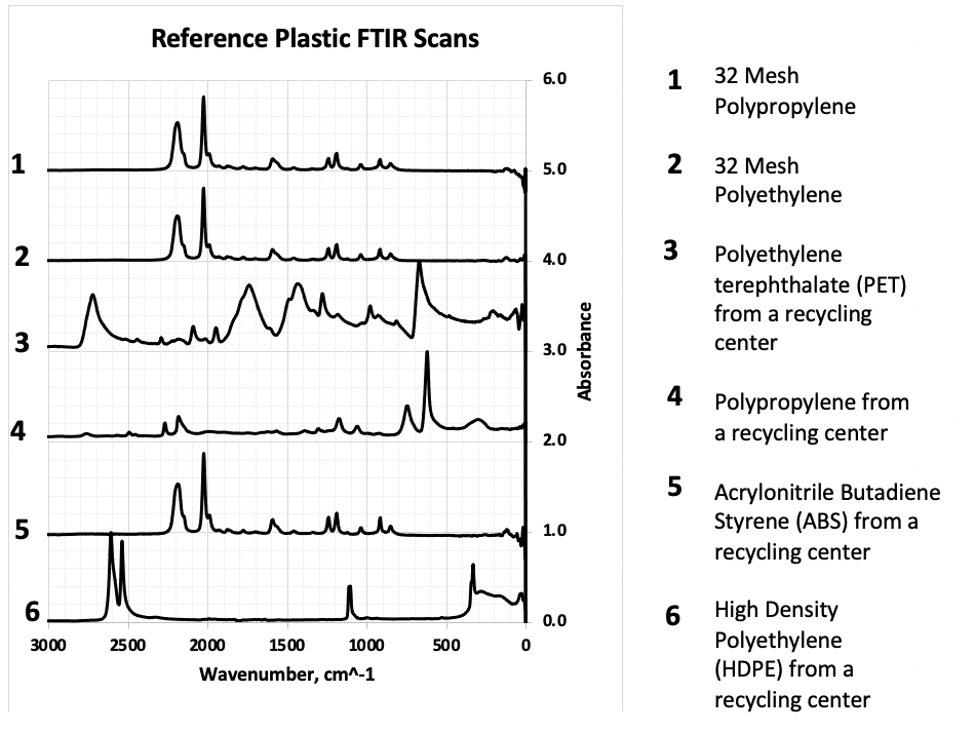
Figure 7. Reference FTIR library
To facilitate polymer identification, known plastic samples were collected from a local recycling center, including Polyethylene terephthalate (PET), Acrylonitrile Butadiene Styrene (ABS), High- density polyethylene (HDPE), PP, and PE. Additionally, 32-mesh PP and PE used as control spikes in the study were analyzed via ATR-FTIR. The resulting spectra were compiled into a reference library for comparative analysis (Figure 7) against the unknown MPs identified in biosolid and biosolid-amended samples.
3. RESULTS
Visual counts of MPs in biosolid samples yielded the following results: Biosolids Trial 1 had 43 MPs per gram of biosolid, and Biosolids Trial 2 had 32 MPs per gram of biosolid (Table 1). In biosolid- amended soils, the microplastic counts were lower, with 20 MPs per gram of biosolid-amended soil in one sample from May 2024 and 28 MPs per gram in another sample from June 2024 (Table 2). In a control field where biosolids were not applied, 8 MPs per gram of soil were counted (Table 2), suggesting the presence of MPs from other anthropogenic sources.
Table 1. Summarized MPs Counts in Biosolids
|
Sample Type |
MPs Count in Biosolids |
|
Biosolids Trial 1 |
43 per gram of biosolid |
|
Biosolids Trial 2 |
32 per gram of biosolid |
Table 2. Summarized MPs Counts in Biosolid – Amended Soils
|
Sample Type |
MPs Count in Biosolid-Amended Soils |
|
Biosolid-Amended Soils from May 2024 |
20 per gram of biosolid-amended soil |
|
Biosolid-Amended Soils from June 2024 |
28 per gram of biosolid-amended soil |
|
Control Soil from a Field Where Biosolids Were Not Applied |
8 per gram of soil |
Visually identified MPs were categorized into fragments, films, foams, fibers, and pellets, as these were the most commonly observed shapes across both biosolids and biosolid-amended soils (Figure 8). The categories of the MPs were selected based on shape and morphology, rather than size, as this approach is consistent with the recommendations of Lusher et al. [23]. Their study suggested dividing MPs into three core morphological groups: bead, fiber, and fragment, with film and foam grouped under the fragment category.
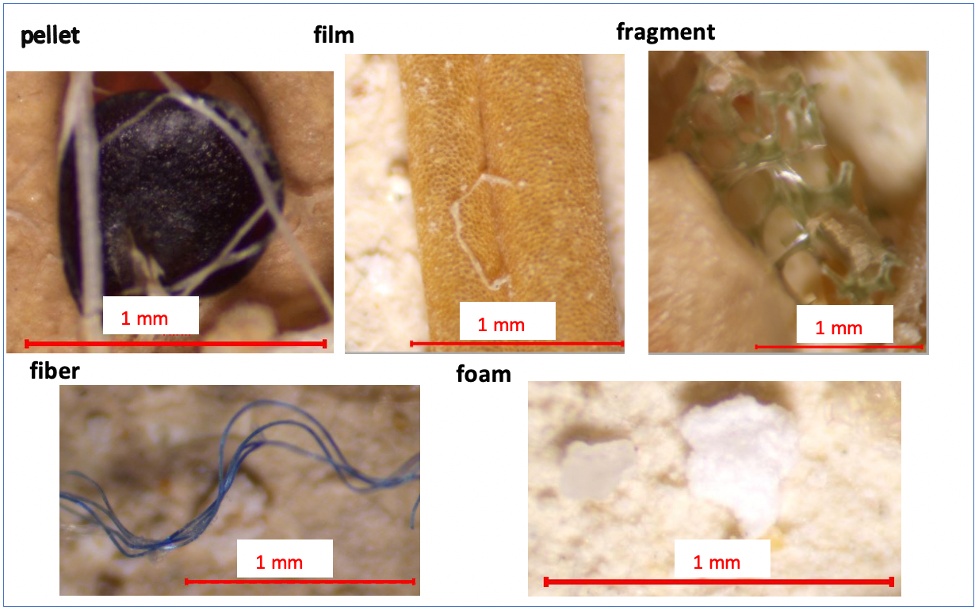
Figure 8. Categorized MPs from biosolids and biosolid-amended soils
Potential sources of error in microplastic counts include misinterpretation of materials or contamination from sources not degraded by Fenton’s Reagent. In biosolid-amended soils, a large volume of opaque fibrous material was observed, raising concerns about the accuracy of MP counts. To investigate whether these materials were plant matter, plant roots from soil samples collected on the university campus in the Wasatch Front were subjected to the digestion and density separation process. Minimal degradation was observed during digestion, and the roots were documented at the end of the process (Figure 9). This suggests that plant roots and other plant matter may influence microplastic counts in biosolid-amended soils. The MP counts in biosolid-amended soils were redone, ensuring the exclusion of materials resembling plant matter. These materials, which appeared more opaque compared to the brighter white or clear fibers, were likely plant material rather than plastic particles (Figure 10). This was further confirmed through ATR-FTIR scans (see Appendix III).
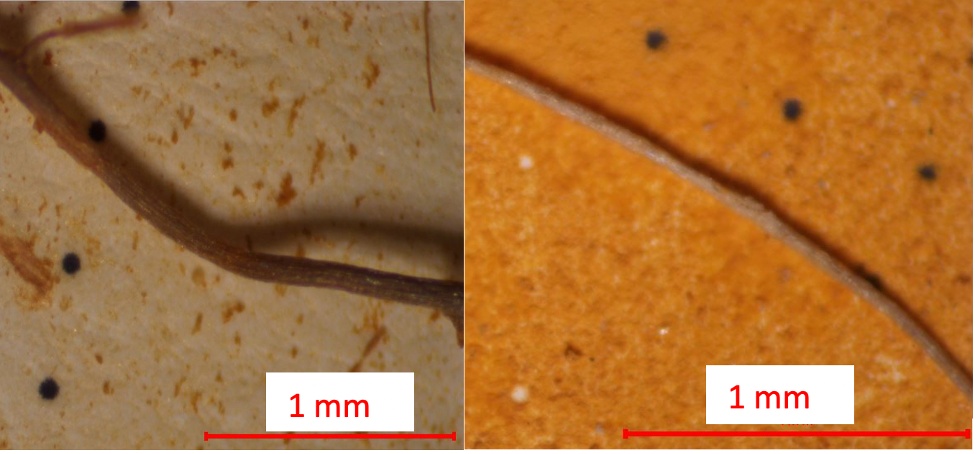
Figure 9. Known plant roots post-digestion and density separation
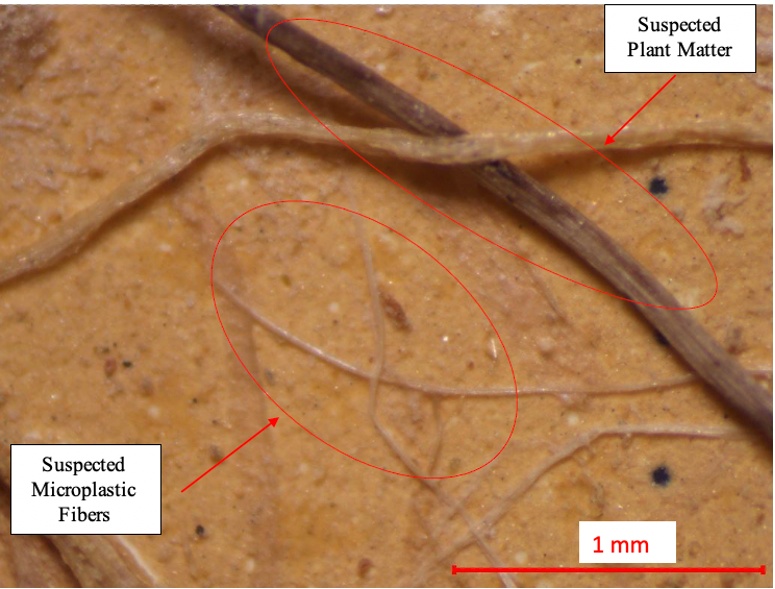
Figure 10. Comparison of suspected plant root contamination and suspected fibrous plastics
ATR FT-IR analysis was conducted as described previously. Due to the scope of this study, it was not feasible to analyze every suspected microplastic. Therefore, a few samples were selected for analysis. One sample, a prominent green suspected microplastic from Biosolids Trial 1 (Figure 11), was scanned. The results did not show distinct similarities to reference spectra (Figure 12), prompting further investigation.

Figure 11. Suspected Microplastic from Biosolids Trial 1
Among the 20 studies reviewed in the work by Xu et al. [24], which examined FTIR and Raman imaging for MPs analysis, some utilized characteristic peaks of functional groups to interpret their data. These studies identified common vibrational bands, including C-H stretching (2980–2780 cm⁻¹), C=O stretching (1760–1670 cm⁻¹), and C-H deformation (1480–1400 cm⁻¹), which are characteristic of organic matter. The removal of organic compounds via Fenton’s reagent suggests that any remaining C- H bonds may indicate the presence of synthetic materials rather than natural organics. In this study, spectral features observed in the 2500–3000 cm⁻¹ region (Figure 12) are interpreted as potential C-H stretching vibrations, which could suggest the presence of synthetic materials. However, this observation remains inconclusive, and further research is required to confirm the nature of the MPs observed, and identify which types of plastic predominate in the counts.
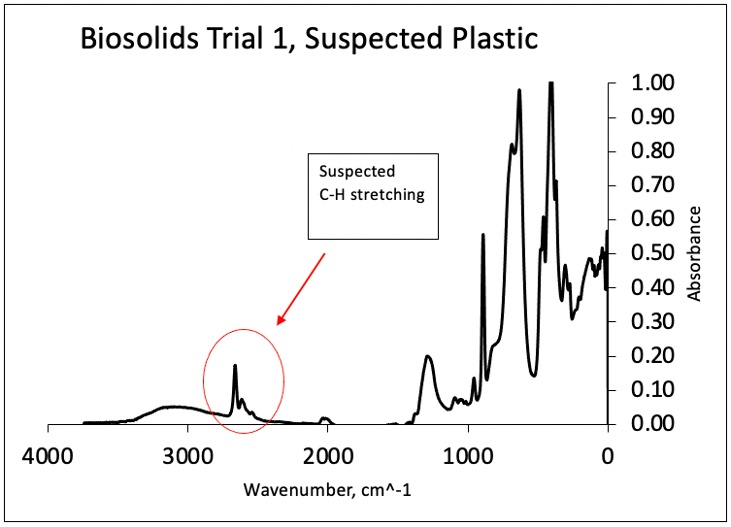
Figure 12. FTIR scan of suspected plastic from Biosolids Trial 1 with suspected C-H stretching in the 2500-3000 cm^-1 region
4. DISCUSSION
A review of 65 studies reporting microplastic concentrations in sludge and biosolids found significant variation, with mean concentrations ranging from 0.193 MPs/gram to 1.69 × 10⁵ MPs/g and a median concentration of 22.4 MPs/g. These discrepancies have been attributed to differences in extraction and quantification methods, highlighting methodological inconsistencies that complicate direct comparisons [21]. Similarly, there is recognition of variability in the visual counts presented in this study. There remains a possibility of misidentification, where particles resembling MPs may have been incorrectly categorized as such. Grid lines on the filters were followed to guide counts, but potential sources of error include double counting and uncertainty introduced by assuming the opposite side of the filter contained the same number of MPs as the counted side. Despite these uncertainties, the measured mean concentration of MPs in biosolids was 37.5 MPs/g, which, considering the observed variability, may fall within a reasonable range. However, this finding contrasts with a study conducted in Southern California introduced earlier, where only 5 MPs per 5 grams of biosolids were reported on average [16]. This suggests that regional differences may influence the levels of MPs retained in biosolids.
Another source of variability arose in the control trials. The control exhibited substantial inconsistency in the amount retained (see Appendix I). Spiked MPs demonstrated a tendency to accumulate along the edges of the glass containers and displayed strong adhesive properties during the removal of the supernatant. To mitigate this issue, pipetting was employed to enhance the transfer efficiency. Additionally, deionized (DI) water was used to dislodge particles adhering to the top of the filtration unit. Despite these efforts, each trial resulted in a variable adjustment factor, affecting the overall counts.
In order to improve the accuracy of counts and reduce variability, if this experiment were to be repeated with additional resources, future work would focus on refining microplastic counting and identification methodologies. Beyond the variability observed in particle counting, the most accurate FTIR scans were obtained by manually picking suspected MPs off the filter. However, this approach is not recommended due to the small size of the particles, which increases the risk of sample loss or contamination. Jia-yu Lin et al. [25] propose leveraging machine learning (ML) and advanced computational techniques to enhance microplastic identification. Specifically, their recommendations include improving ML algorithms, developing a comprehensive database of microplastic structural characteristics for model training, and integrating ML with conventional methods such as image analysis. These advancements have the potential to enhance both the physical and chemical characterization of MPs, thereby improving detection accuracy and efficiency.
Previous studies have shown that microplastic concentrations in biosolid-amended soils vary, with some fields exhibiting increased microplastic levels following biosolid application. However, these studies also indicate that soils often contain pre-existing microplastic contamination prior to biosolid- amendment [26]. In this study, microplastic concentrations were measured at 20 and 28 MPs per gram in biosolid-amended soils, while the control soil contained 8 MPs per gram. These findings support the notion that MPs are often present in soil before biosolid application and suggest that both pre-existing contamination and biosolid amendments may contribute to observed microplastic levels.
The earlier Washington case study revealed a microplastic concentration of 0.544 MP/g in biosolid-amended soils [17], notably lower than the average concentration of 24 MP/g found in the biosolid-amended soils of the current study. While this difference could suggest overcounting or other sources of error, it may also indicate that the microplastic concentrations in the soils measured in this study are indeed higher, potentially due to site-specific factors or methodological differences. The Washington study also reported a higher concentration in the biosolids themselves (12,200 MPs/kg, or 12.2 MPs/g) [17], which aligns with the current study’s findings, where the mean MP concentration in biosolids (37.5 MPs/g) was higher than the mean MP concentration in the biosolid-amended soils (24 MPs/g).
The presence of MPs in control soils raises questions about their anthropogenic sources. MPs in agricultural soils can originate from a variety of human activities, including farming practices, irrigation, and airborne particles. These MPs break down through processes such as sunlight exposure and mechanical wear, and can move through the soil, influenced by soil type and farming activities [27]. Another potential source is fibers released from washing machines. A study estimated that a typical 6 kg load of synthetic materials could release between 137,951 and 728,789 fibers per wash, depending on the material type [28]. This study also relays that fibers have been reported in effluent from sewage treatment plants, which could enter runoff and ultimately reach agricultural fields or aquatic habitats.
Given the widespread presence of MPs in both biosolids and soils in various studies globally, as well as my own observations along the Wasatch Front, this raises questions about whether the benefits of biosolid application outweigh its drawbacks or if limitations should be considered. Biosolids have notable benefits, including their contribution to renewable energy production, such as biogas, and their use in civil engineering applications like landfill capping and construction materials. Furthermore, their land application provides economic and waste management advantages, such as conserving landfill space, reducing the demand for non-renewable resources like phosphorus, and decreasing reliance on synthetic fertilizers [29].
However, perspectives on their use vary. One author argues that the harm caused by MPs in biosolids outweighs the benefits of their current use in agriculture. They propose a global mandate to incorporate at least 7% biosolids into the production of clay bricks, which would help mitigate environmental damage, reduce greenhouse gas emissions, and prevent further contamination of agricultural soils [30]. Another study believes that there should be more investigation into the sources and movement of MPs in agricultural soils, suggesting that while biosolids may contribute to soil contamination, other factors such as the use of plastic materials in farming (e.g., plastic films, irrigation pipes) and MPs deposited from the atmosphere also play significant roles in the overall microplastic load [31]. These varying perspectives highlight the complexity of managing biosolid applications in agriculture.
In conclusion, this study contributes important data by confirming the presence of MPs in both biosolids and soils within the Wasatch Front region, thereby establishing a foundational database for future research. The findings underscore the need for further investigation into the extent of microplastic contamination and the potential implications for environmental and public health along the Wasatch Front. With this knowledge, it is crucial to consider the possibility of more stringent regulations regarding microplastic management and to recognize the pervasive presence of MPs in our ecosystems.
5. CONCLUSION
Overall, MPs are a persistent environmental issue that should be monitored in both wastewater and soils across the Wasatch Front. From a local report, biosolid-amended soils are applied at a rate of 7.8 tons per acre per year [1]. Using the average value of the biosolid-amended soils microplastic counts (Table 2), it is evident that approximately 9.4 x 108 MPs will be introduced into agricultural soils over 5 years. (see Appendix IV). Biosolids that are sold as fertilizer to consumers may also contain MPs, which can accumulate in gardens. This local study is crucial for understanding impacts of microplastics in the Wasatch front region and supporting informed management and policy decisions.
6. APPENDICES
Appendix I: Recovery Efficiency Adjustments for Control Sample
This section outlines the control mass balance equation and provides an example calculation for the biosolids trial, along with adjusted microplastic (MP) counts for various samples.
Control Mass Balance Equation:
Mass of spiked PP and PE − ((Mass of retained MPs + Filter weight) − Filter weight)
= Amount of MPs retained in the control
Example Calculation for Biosolids Trial 2:
- Mass of PP: 0.10 g Mass of PE: 0.10 g
- Mass of Filter: 0.079 g
- Mass of retained MPs + Filter weight: 0.173g
0.20𝑔 − (0.173 − 0.079)𝑔 = 0.106𝑔
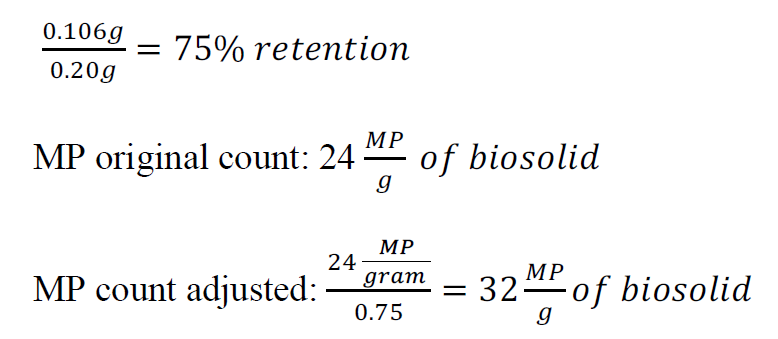
Table 3: MP counts adjusted
|
Sample Type |
Percentage Retained from Control |
Original Count (MP/g) |
Reported Count (MP/g) |
|
Biosolids Trial 1 |
7% |
3 |
43 |
|
Biosolids Trial 2 |
75% |
24 |
32 |
|
Biosolid-Amended Soils from May 2024 |
85% |
17 |
20 |
|
Biosolid-Amended Soils from June 2024 |
80% |
22 |
28 |
|
Control Soil from Field where Biosolids were not Applied |
90% |
7 |
8 |
Appendix II: Additional Microplastics FTIR Scans
This appendix includes FTIR scan data for additional microplastic samples, displaying the image of suspected plastics followed by the corresponding spectral graph.
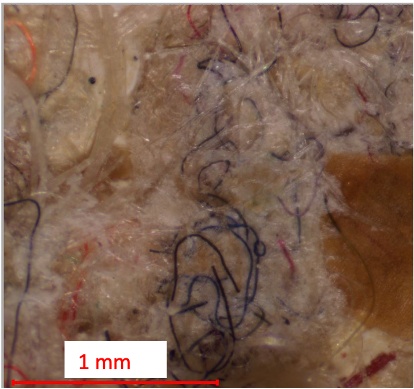
Figure 13. Suspected fibrous plastics from Biosolids Trial 1
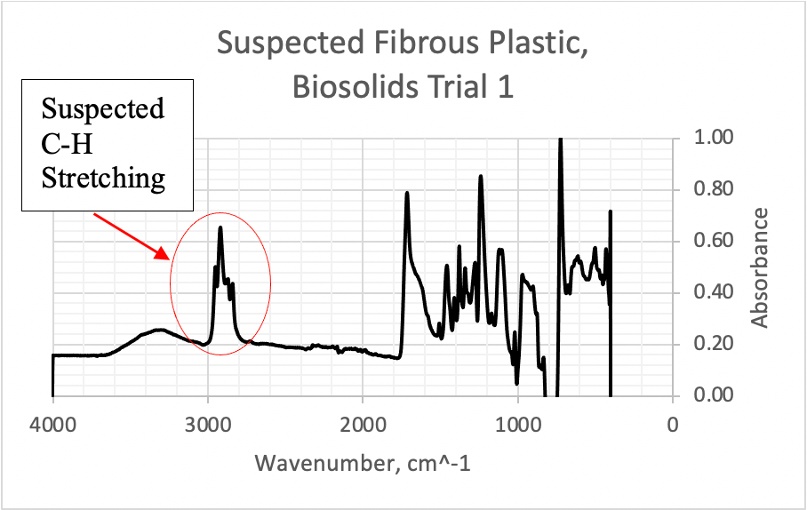
Figure 14. FTIR scan of one suspected fibrous plastic from Biosolids Trial 1 with suspected C- H stretching 2500-3000 cm^-1 region
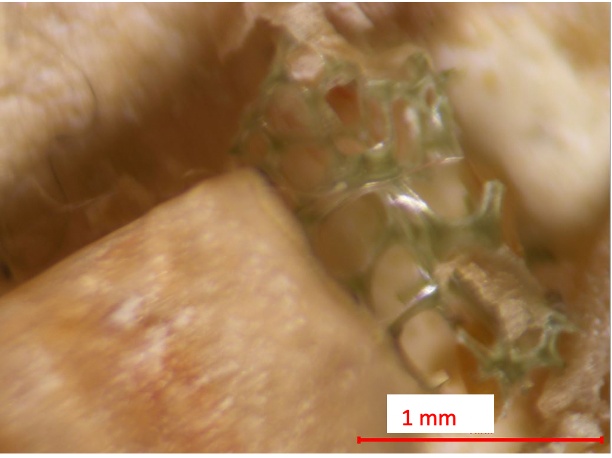
Figure 15. Suspected fragment from Biosolids Trial 1
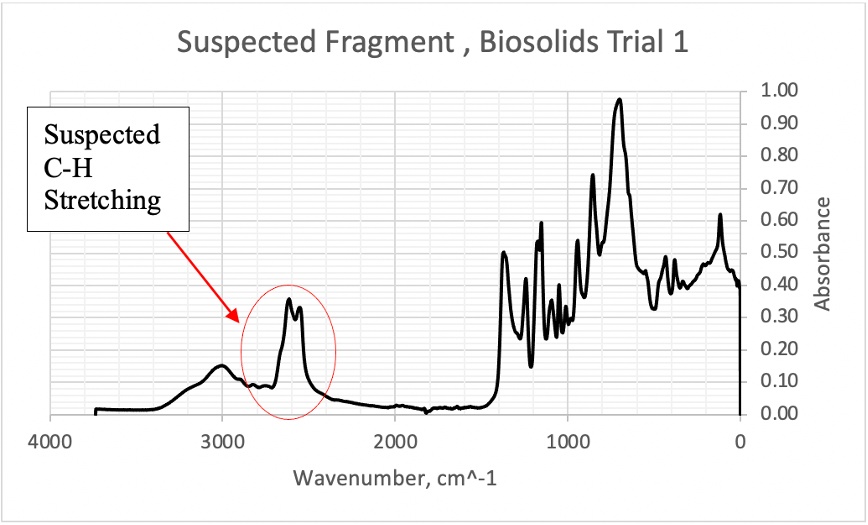
Figure 16. FTIR scan of suspected fragment from Biosolids Trial 1 with suspected C-H stretching 2500-3000 cm^-1 region
Appendix III: FTIR Root Scans
This appendix presents FTIR root scan data, featuring both the FTIR spectral graphs and corresponding images of plant roots. These figures provide insight into how plant root contamination was identified in biosolid-amended soils.

Figure 17. Plant roots sourced from university campus soil
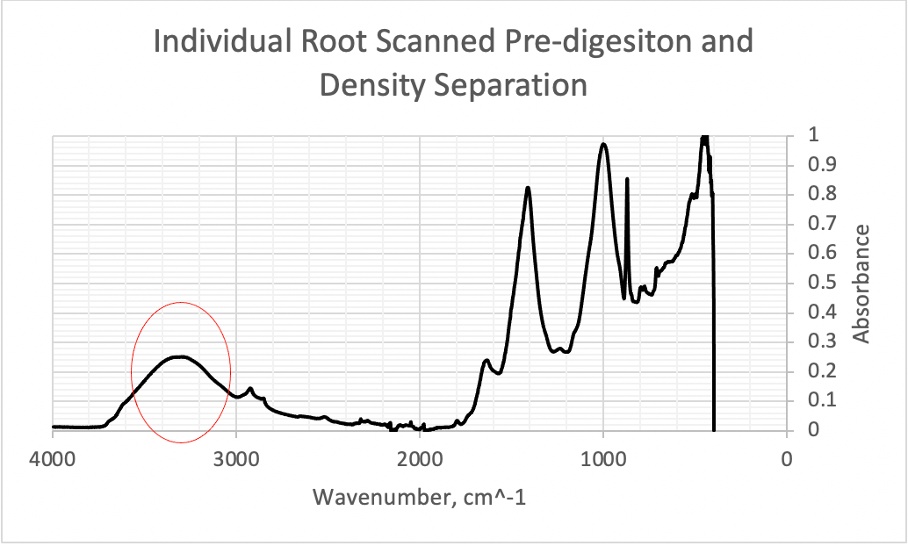
Figure 18. An isolated plant root from plant roots sourced from university campus soil with a broad peak between 3000-4000 cm-1
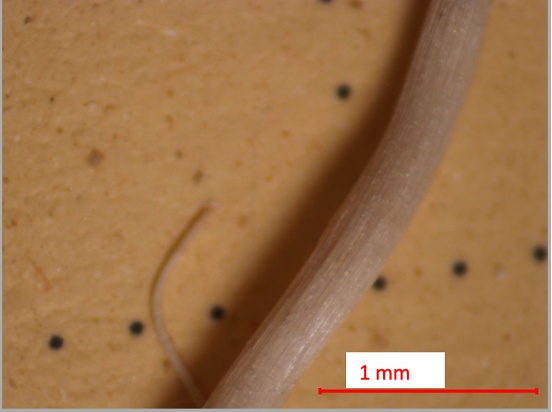
Figure 19. Suspected root in May 2024 Biosolid-Amended Soils
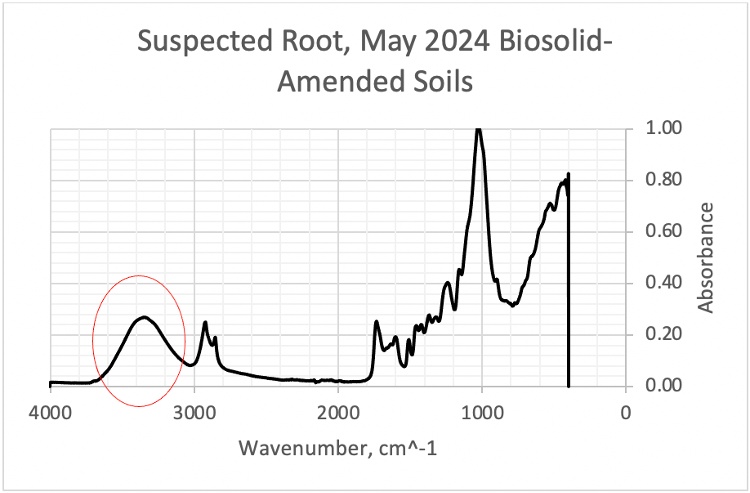
Figure 20. FTIR scan of a suspected root in May 2024 biosolid-amended soils, showing a broad peak in the 3000-4000 cm⁻¹ region, similar to the spectral pattern of an individual root scanned pre and post-digestion and density separation.

Figure 21. Individual root post-digestion and density separation
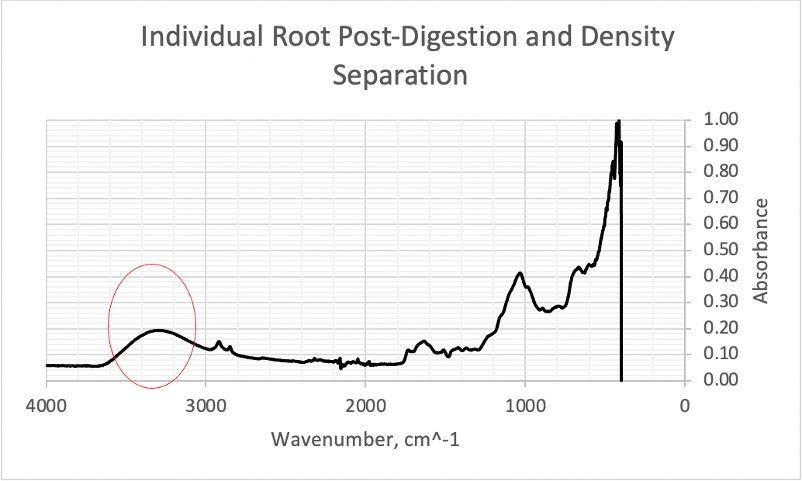
Figure 22. Individual root post digestion and density separation with a broad peak in the 3000- 4000cm-1region.
Appendix IV: Total MPs Applied to Agricultural Soils Over Five Years in the Wasatch Front
This appendix estimates the total MPs added to agricultural soils in the Wasatch Front region over five years. With 7.8 tons of biosolids applied per acre annually [1], the following analysis quantifies the associated MP deposition.
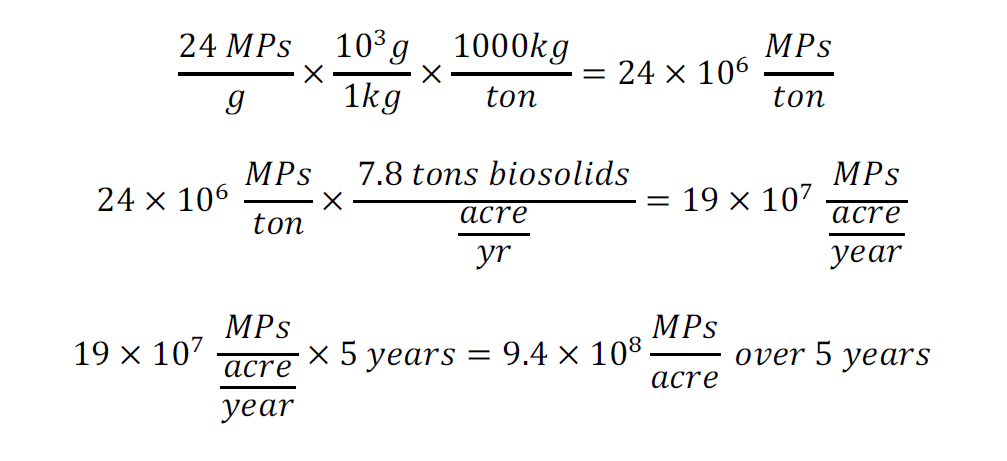
ACKNOWLEDGEMENTS
The author wishes to express sincere gratitude to Dr. Jennifer Weidhaas for her mentorship and for providing this opportunity, as well as to the University of Utah Department of Civil and Environmental Engineering for their support. This project was funded by the University of Utah Office of Undergraduate Research, with additional financial support from the Department of Civil and Environmental Engineering.
REFERENCES
- Adhikari K, Pearce CI, Sanguinet KA, Bary AI, Chowdhury I, Eggleston I, Xing B, Flury M. Accumulation of microplastics in soil after long-term application of biosolids and atmospheric deposition. Science of The Total Environment. 2024;912:168883. doi:10.1016/j.scitotenv.2023.168883
- Andrady AL. The plastic in microplastics: A review. Marine Pollution Bulletin. 2017;119(1):12–22. doi:10.1016/j.marpolbul.2017.01.082
- Biosolids report from a water treatment facility in the Wasatch Front, 2019-2024.
- Boots B, Russell CW, Green DS. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environmental Science & Technology. 2019;53(19):11496–11506. doi:10.1021/acs.est.9b03304
- Carr SA, Liu J, Tesoro AG. Transport and fate of microplastic particles in wastewater treatment plants. Water Research. 2016;91:174–182. doi:10.1016/j.watres.2016.01.002
- Circelli L, Cheng Z, Garwood E, Yuksel K, Di Iorio E, Angelico R, Colombo C. Comparison of ATR-FTIR and NIR spectroscopy for identification of microplastics in biosolids. Science of The Total Environment. 2024;916:170215. doi:10.1016/j.scitotenv.2024.170215
- Crossman J, Hurley RR, Futter M, Nizzetto L. Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Science of The Total Environment. 2020;724:138334. doi:10.1016/j.scitotenv.2020.138334
- Dey TK, Uddin MdE, Jamal M. Detection and removal of microplastics in wastewater: evolution and impact. Environmental Science and Pollution Research. 2021;28(14):16925–16947. doi:10.1007/s11356-021-12943-5
- Dokl M, Copot A, Krajnc D, Fan YV, Vujanović A, Aviso KB, Tan RR, Kravanja Z, Čuček L. Global projections of plastic use, end-of-life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050. Sustainable Production and Consumption. 2024;51:498–518. doi:10.1016/j.spc.2024.09.025
- Elgarahy AM, Eloffy MG, Priya AK, Yogeshwaran V, Yang Z, Elwakeel KZ, Lopez-Maldonado EA. Biosolids management and utilizations: A review. Journal of Cleaner Production. 2024;451:141974. doi:10.1016/j.jclepro.2024.141974
- Harley-Nyang D, Memon FA, Osorio Baquero A, Galloway T. Variation in microplastic concentration, characteristics and distribution in sewage sludge & biosolids around the world. Science of The Total Environment. 2023;891:164068. doi:10.1016/j.scitotenv.2023.164068
- Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environmental Science & Technology. 2019;53(3):1039–1047. doi:10.1021/acs.est.8b05297
- Henze M, Van Loosdrecht MCM, Ekama GA, Brdjanovic D. Biological Wastewater Treatment: Principles, Modelling and Design. IWA Publishing; 2008 [accessed 2025 Apr 10]. http://iwaponline.com/ebooks/book/59/Biological-Wastewater-Treatment-Principles. doi:10.2166/9781780401867
- Koutnik VS, Alkidim S, Leonard J, DePrima F, Cao S, Hoek EMV, Mohanty SK. Unaccounted Microplastics in Wastewater Sludge: Where Do They Go? ACS ES&T Water. 2021;1(5):1086–1097. doi:10.1021/acsestwater.0c00267
- Li Q, Wu J, Zhao X, Gu X, Ji R. Separation and identification of microplastics from soil and sewage sludge. Environmental Pollution. 2019;254:113076. doi:10.1016/j.envpol.2019.113076
- Lin J, Liu H, Zhang J. Recent advances in the application of machine learning methods to improve identification of the microplastics in environment. Chemosphere. 2022;307:136092. doi:10.1016/j.chemosphere.2022.136092
- Lusher AL, Bråte ILN, Munno K, Hurley RR, Welden NA. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Applied Spectroscopy. 2020;74(9):1139–1153. doi:10.1177/0003702820930733
- Mohajerani A, Karabatak B. Microplastics and pollutants in biosolids have contaminated agricultural soils: An analytical study and a proposal to cease the use of biosolids in farmlands and utilise them in sustainable bricks. Waste Management. 2020;107:252–265. doi:10.1016/j.wasman.2020.04.021
- Napper IE, Thompson RC. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Marine Pollution Bulletin. 2016;112(1–2):39–45. doi:10.1016/j.marpolbul.2016.09.025
- Prata JC. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Marine Pollution Bulletin. 2018;129(1):262–265. doi:10.1016/j.marpolbul.2018.02.046
- Radford F, Horton A, Hudson M, Shaw P, Williams I. Agricultural soils and microplastics: Are biosolids the problem? Frontiers in Soil Science. 2023;2:941837. doi:10.3389/fsoil.2022.941837
- Rochman CM, Brookson C, Bikker J, Djuric N, Earn A, Bucci K, Athey S, Huntington A, McIlwraith H, Munno K, et al. Rethinking microplastics as a diverse contaminant suite. Environmental Toxicology and Chemistry. 2019;38(4):703–711. doi:10.1002/etc.4371
- Ruffell H, Pantos O, Robinson B, Gaw S. A method for the extraction of microplastics from solid biowastes including biosolids, compost, and soil for analysis by µ-FTIR. MethodsX. 2024;12:102761. doi:10.1016/j.mex.2024.102761
- SAB Advisory on Aquatic LifeWater Quality Criteria for Contaminates of Emerging Concern. 2008. https://www.epa.gov/sites/default/files/2015-08/documents/sab_advisory_on_aquatic_life_wqc_for_contaminants_of_emerging_concern.pdf
- Schütze B, Thomas D, Kraft M, Brunotte J, Kreuzig R. Comparison of different salt solutions for density separation of conventional and biodegradable microplastic from solid sample matrices. Environmental Science and Pollution Research. 2022;29(54):81452–81467. doi:10.1007/s11356-022-21474-6
- Tariq M, Iqbal B, Khan I, Khan AR, Jho EH, Salam A, Zhou H, Zhao X, Li G, Du D. Microplastic contamination in the agricultural soil—mitigation strategies, heavy metals contamination, and impact on human health: a review. Plant Cell Reports. 2024;43(3):65. doi:10.1007/s00299-024-03162-6
- Tian L, Jinjin C, Ji R, Ma Y, Yu X. Microplastics in agricultural soils: sources, effects, and their fate. Current Opinion in Environmental Science & Health. 2022;25:100311. doi:10.1016/j.coesh.2021.100311
- Wael H, Vanessa EB, Mantoura N, Antonios DE. Tiny pollutants, big consequences: investigating the influence of nano- and microplastics on soil properties and plant health with mitigation strategies. Environmental Science: Processes & Impacts. 2025:10.1039.D4EM00688G. doi:10.1039/D4EM00688G
- Xu J-L, Thomas KV, Luo Z, Gowen AA. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends in Analytical Chemistry. 2019;119:115629. doi:10.1016/j.trac.2019.115629
- Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JKH, Wu C, Lam PKS. Understanding plastic degradation and microplastic formation in the environment: A review. Environmental Pollution. 2021;274:116554. doi:10.1016/j.envpol.2021.116554
Biosolids report from a water treatment facility in the Wasatch Front, 2019-2024.
H. Wael, E. B. Vanessa, N. Mantoura, and D. E. Antonios, “Tiny pollutants, big consequences: investigating the influence of nano- and microplastics on soil properties and plant health with mitigation strategies,” Environ. Sci. Process. Impacts, p. 10.1039.D4EM00688G, 2025, doi: 10.1039/D4EM00688G.
M. Tariq et al., “Microplastic contamination in the agricultural soil—mitigation strategies, heavy metals contamination, and impact on human health: a review,” Plant Cell Rep., vol. 43, no. 3, p. 65, Mar. 2024, doi: 10.1007/s00299-024-03162-6.
M. Dokl et al., “Global projections of plastic use, end-of-life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050,” Sustain. Prod. Consum., vol. 51, pp. 498–518, Nov. 2024, doi: 10.1016/j.spc.2024.09.025.
K. Zhang et al., “Understanding plastic degradation and microplastic formation in the environment: A review,” Environ. Pollut., vol. 274, p. 116554, Apr. 2021, doi: 10.1016/j.envpol.2021.116554.
N. B. Hartmann et al., “Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris,” Environ. Sci. Technol., vol. 53, no. 3,
pp. 1039–1047, Feb. 2019, doi: 10.1021/acs.est.8b05297.
C. M. Rochman et al., “Rethinking microplastics as a diverse contaminant suite,” Environ. Toxicol. Chem., vol. 38, no. 4, pp. 703–711, Apr. 2019, doi: 10.1002/etc.4371.
A. L. Andrady, “The plastic in microplastics: A review,” Mar. Pollut. Bull., vol. 119, no. 1, pp. 12–22, Jun. 2017, doi: 10.1016/j.marpolbul.2017.01.082.
M. Henze, M. C. M. Van Loosdrecht, G. A. Ekama, and D. Brdjanovic, Biological Wastewater Treatment: Principles, Modelling and Design. IWA Publishing, 2008. doi: 10.2166/9781780401867.
J. C. Prata, “Microplastics in wastewater: State of the knowledge on sources, fate and solutions,” Mar. Pollut. Bull., vol. 129, no. 1, pp. 262–265, Apr. 2018, doi: 10.1016/j.marpolbul.2018.02.046.
V. S. Koutnik et al., “Unaccounted Microplastics in Wastewater Sludge: Where Do They Go?,” ACS EST Water, vol. 1, no. 5, pp. 1086–1097, May 2021, doi: 10.1021/acsestwater.0c00267.
SAB Advisory on Aquatic LifeWater Quality Criteria for Contaminates of Emerging Concern.” U.S. Envrionmental Protection Agency, Science Advisory Board, Dec. 18, 2008. [Online]. Available: https://www.epa.gov/sites/default/files/2015- 08/documents/sab_advisory_on_aquatic_life_wqc_for_contaminants_of_emerging_concern.pdf
J. Crossman, R. R. Hurley, M. Futter, and L. Nizzetto, “Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment,” Sci. Total Environ., vol. 724, p. 138334, Jul. 2020, doi: 10.1016/j.scitotenv.2020.138334.
B. Boots, C. W. Russell, and D. S. Green, “Effects of Microplastics in Soil Ecosystems: Above and Below Ground,” Environ. Sci. Technol., vol. 53, no. 19, pp. 11496–11506, Oct. 2019, doi: 10.1021/acs.est.9b03304.
T. K. Dey, Md. E. Uddin, and M. Jamal, “Detection and removal of microplastics in wastewater: evolution and impact,” Environ. Sci. Pollut. Res., vol. 28, no. 14, pp. 16925–16947, Apr. 2021, doi: 10.1007/s11356-021-12943-5.
S. A. Carr, J. Liu, and A. G. Tesoro, “Transport and fate of microplastic particles in wastewater treatment plants,” Water Res., vol. 91, pp. 174–182, Mar. 2016, doi: 10.1016/j.watres.2016.01.002.
K. Adhikari et al., “Accumulation of microplastics in soil after long-term application of biosolids and atmospheric deposition,” Sci. Total Environ., vol. 912, p. 168883, Feb. 2024, doi: 10.1016/j.scitotenv.2023.168883.
Q. Li, J. Wu, X. Zhao, X. Gu, and R. Ji, “Separation and identification of microplastics from soil and sewage sludge,” Environ. Pollut., vol. 254, p. 113076, Nov. 2019, doi: 10.1016/j.envpol.2019.113076.
H. Ruffell, O. Pantos, B. Robinson, and S. Gaw, “A method for the extraction of microplastics from solid biowastes including biosolids, compost, and soil for analysis by µ-FTIR,” MethodsX, vol. 12, p. 102761, Jun. 2024, doi: 10.1016/j.mex.2024.102761.
B. Schütze, D. Thomas, M. Kraft, J. Brunotte, and R. Kreuzig, “Comparison of different salt solutions for density separation of conventional and biodegradable microplastic from solid sample matrices,” Environ. Sci. Pollut. Res., vol. 29, no. 54, pp. 81452–81467, Nov. 2022, doi: 10.1007/s11356-022-21474-6.
D. Harley-Nyang, F. A. Memon, A. Osorio Baquero, and T. Galloway, “Variation in microplastic concentration, characteristics and distribution in sewage sludge & biosolids around the world,” Sci. Total Environ., vol. 891, p. 164068, Sep. 2023, doi: 10.1016/j.scitotenv.2023.164068.
L. Circelli et al., “Comparison of ATR-FTIR and NIR spectroscopy for identification of microplastics in biosolids,” Sci. Total Environ., vol. 916, p. 170215, Mar. 2024, doi: 10.1016/j.scitotenv.2024.170215.
A. L. Lusher, I. L. N. Bråte, K. Munno, R. R. Hurley, and N. A. Welden, “Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization,” Appl. Spectrosc., vol. 74, no. 9,
pp. 1139–1153, Sep. 2020, doi: 10.1177/0003702820930733.
J.-L. Xu, K. V. Thomas, Z. Luo, and A. A. Gowen, “FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects,” TrAC Trends Anal. Chem., vol. 119,
p. 115629, Oct. 2019, doi: 10.1016/j.trac.2019.115629.
J. Lin, H. Liu, and J. Zhang, “Recent advances in the application of machine learning methods to improve identification of the microplastics in environment,” Chemosphere, vol. 307, p. 136092, Nov. 2022, doi: 10.1016/j.chemosphere.2022.136092.
J. Crossman, R. R. Hurley, M. Futter, and L. Nizzetto, “Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment,” Sci. Total Environ., vol. 724, p. 138334, Jul. 2020, doi: 10.1016/j.scitotenv.2020.138334.
L. Tian, C. Jinjin, R. Ji, Y. Ma, and X. Yu, “Microplastics in agricultural soils: sources, effects, and their fate,” Curr. Opin. Environ. Sci. Health, vol. 25, p. 100311, Feb. 2022, doi: 10.1016/j.coesh.2021.100311.
I. E. Napper and R. C. Thompson, “Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions,” Mar. Pollut. Bull., vol. 112, no. 1–2, pp. 39–45, Nov. 2016, doi: 10.1016/j.marpolbul.2016.09.025.
A. M. Elgarahy et al., “Biosolids management and utilizations: A review,” J. Clean. Prod., vol. 451, p. 141974, Apr. 2024, doi: 10.1016/j.jclepro.2024.141974.
A. Mohajerani and B. Karabatak, “Microplastics and pollutants in biosolids have contaminated agricultural soils: An analytical study and a proposal to cease the use of biosolids in farmlands and utilise them in sustainable bricks,” Waste Manag., vol. 107, pp. 252–265, Apr. 2020, doi: 10.1016/j.wasman.2020.04.021.
F. Radford, A. Horton, M. Hudson, P. Shaw, and I. Williams, “Agricultural soils and microplastics: Are biosolids the problem?,” Front. Soil Sci., vol. 2, p. 941837, Jan. 2023, doi: 10.3389/fsoil.2022.941837.
Media Attributions
- Figure 1
- Figure 2
- Figure 3
- Figure 4
- Figure 5
- Figure 6
- Figure 7
- Figure 8
- Figure 9
- Figure 10
- Figure 11
- Figure 12
- Capture
- Figure 13
- Figure 14
- Figure 15
- Figure 16
- Figure 17
- Figure 18
- Figure 19
- Figure 20
- Figure 21
- Figure 22
- Capture

