Spencer Fox Eccles School of Medicine
60 The Relationship of Demographics to Adverse Events Following Immunization Within the Utah BEEHIVE Study Population
Tyler Allison; Danika Nakai; Feiyun Yan; Joshua Griffin; Jacob Mckell; Hongwei Zhao; Andrew L. Phillips; German L. Ellsworth; Matthew S. Thiese; and Sarang K. Yoon
Faculty Mentor: Sarang K. Yoon (Occupational and Environmental Health, University of Utah)
Introduction
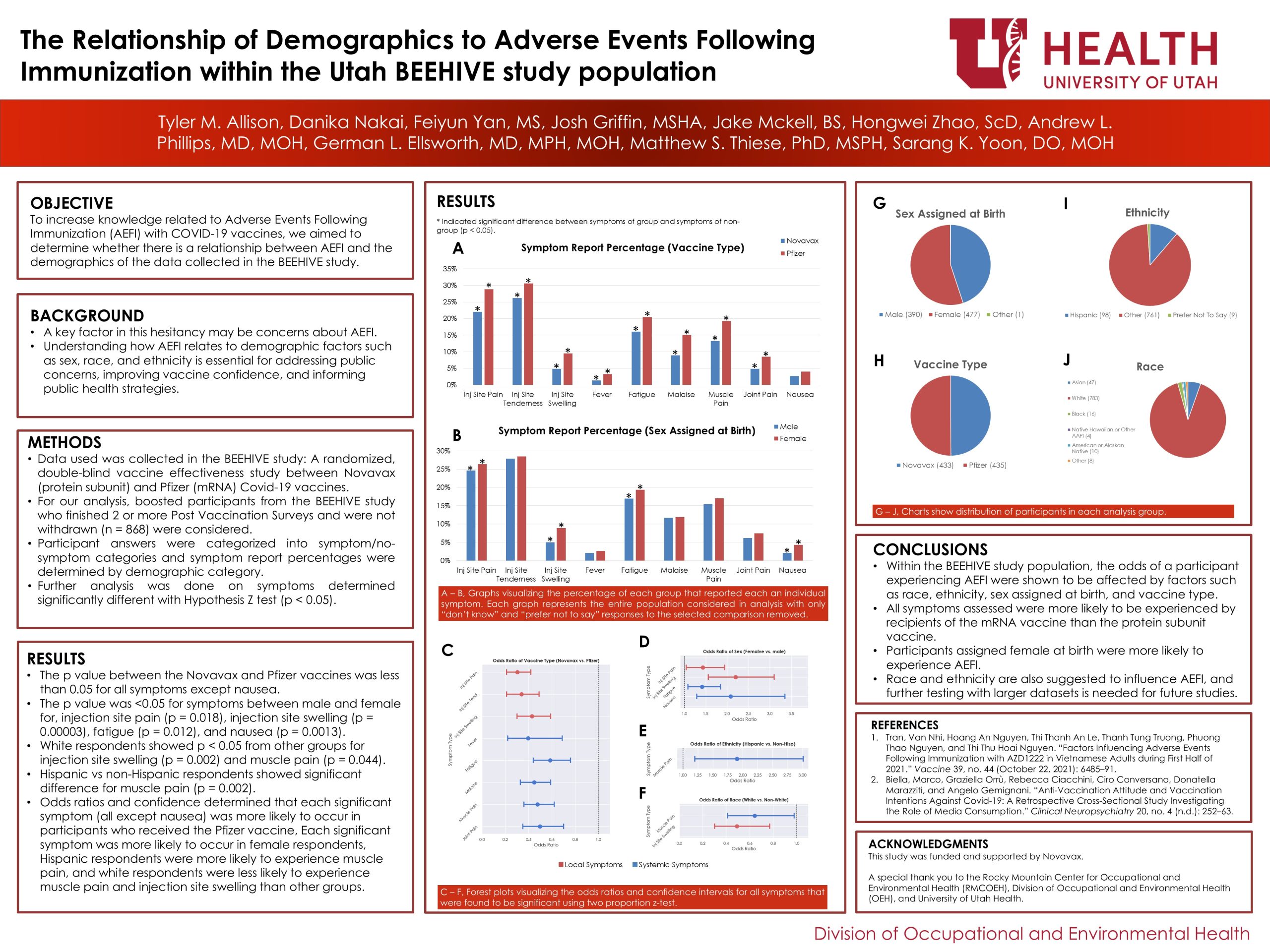
Misinformation surrounding COVID-19 vaccines has contributed to public scepticism, particularly regarding vaccine safety and potential adverse effects.[2] Anti-vaccine narratives have lowered vaccination intent among those susceptible to such messaging.[2] Concerns about Adverse Events Following Immunization (AEFI) may be a key factor in this hesitancy. Understanding how AEFI relates to demographic factors such as sex, race, and ethnicity is essential for addressing public concerns, improving vaccine confidence, and informing public health strategies. To increase knowledge related to AEFI of COVID-19 vaccines, we aimed to determine whether there is a relationship between AEFI, and the demographic factors using data collected in the BEEHIVE study.
Methods
Data used was collected in the BEEHIVE study: A randomized, double-blind vaccine efficacy study. Participants were recruited from the Greater Salt Lake City area from Nov 2023 to Mar 2024. Vaccinated participants were randomized 1:1 into either the Novavax (protein subunit) or the Pfizer (mRNA) booster groups. Participants completed an online enrolment survey, and an in-person vaccination visit. Booster recipients were monitored for adverse events, completing post-vaccination surveys (PVS) on Days 1, 2, and 7. For our analysis, boosted participants who finished 2 PVS (n=868) and were not withdrawn were considered. PVS answers were categorized to symptom/no-symptom groups and answers were sorted into demographic and vaccination type categories. Each group’s percentage answer to each symptom was determined by normalizing the number of each response by the group size. Additional analysis was performed on symptoms shown to have a p-value less than 0.05 determined by a hypothesis z-test between group and non-group responses. Odds ratios and confidence intervals were determined for these cases by comparing the answers of respondents categorized into that group against the respondents categorized in all other groups.
Results
In the vaccine comparison, the difference between the Novavax (n = 432) and Pfizer (n = 435) vaccines was significant for all symptoms except for nausea. In the race and ethnicity categories, white respondents (n = 783) showed significant difference from non-white groups for injection site swelling (p = 0.002) and muscle pain (p = 0.044). Hispanic respondents (n = 98) showed significant difference from non-Hispanic groups for muscle pain (p =0.01). In the sex assigned at birth comparison, the difference in symptoms between male (n = 390) and female (n = 477) was significant for injection site swelling (p = 0.00008), fatigue (p = 0.04), and nausea (p = 0.003). See figures A – B and G – J. Odds ratios and confidence intervals for significant symptoms determined the following: each symptom recorded except for nausea was more likely to occur in participants who received the Pfizer vaccine, Female respondents were more likely to experience injection site pain, injection site swelling, fatigue, and nausea, Hispanic respondents were more likely to experience muscle pain than non-Hispanic participants and white respondents were less likely to experience muscle pain and injection site swelling than other groups. See figures C – F.
Conclusions
Within the BEEHIVE study population, AEFI was shown to be affected by race, ethnicity, sex assigned at birth, and vaccine type. The sweeping differences in AEFI likelihood shown between the Novavax (protein subunit) and Pfizer (mRNA) vaccines suggest that AEFI is less commonly experienced by individuals who receive the Novavax vaccine compared with the Pfizer vaccine. Similar conclusions can also be drawn between female and male participants, suggesting that participants assigned female at birth are more likely to experience AEFI. Race and ethnicity are also suggested to influence AEFI, and further testing with larger datasets is needed for future studies.
Bibliography
Biella, Marco, Graziella Orrù, Rebecca Ciacchini, Ciro Conversano, Donatella Marazziti, and Angelo Gemignani. “Anti-Vaccination Attitude and Vaccination Intentions Against Covid-19: A Retrospective Cross-Sectional Study Investigating the Role of Media Consumption.” Clinical Neuropsychiatry 20, no. 4 (n.d.): 252–63.
Tran, Van Nhi, Hoang An Nguyen, Thi Thanh An Le, Thanh Tung Truong, Phuong Thao Nguyen, and Thi Thu Hoai Nguyen. “Factors Influencing Adverse Events Following Immunization with AZD1222 in Vietnamese Adults during First Half of 2021.” Vaccine 39, no. 44 (October 22, 2021): 6485–91.
Werner, Felix, Nikoletta Zeschick, Thomas Kühlein, Philipp Steininger, Klaus Überla, Isabelle Kaiser, Maria Sebastião, Susann Hueber, and Lisette Warkentin. “Patient-Reported Reactogenicity and Safety of COVID-19 Vaccinations vs. Comparator Vaccinations: A Comparative Observational Cohort Study.” BMC Medicine 21, no. 1 (September 19, 2023): 358. https://doi.org/10.1186/s12916-023-03064-6.
Media Attributions
- AEFI Poster 2025 JPEG

