John and Marcia Price College of Engineering
31 GJA1-20K Therapy Increases Renal Corical ATP Concentration in Pig Model of Severe Ischemia-Reperfusion Injury
Ryleigh Smith
Faculty Mentor: Guillaume Hoareau (Physiology, University of Utah)
ABSTRACT
GJA1-20k is an endogenous mammalian peptide. GJA1-20k exerts protective cellular effects and its expression increases in tissues undergoing ischemic stress. The protective effects of GJA1-20k after ischemia are multimodal. It is already known that GJA1-20k interacts with actin surrounding the mitochondria, causing mitochondrial fission through a novel pathway. This peptide also protects mitochondria undergoing oxidative stress by decreasing oxygen consumption, though the mechanism is not understood. The protective effects of GJA1-20k are critical to the survival of tissues experiencing ischemia since mitochondria regulate cell apoptosis and can release damaging agents such as reactive oxygen species and mitochondrial DNA. This study investigates several therapeutic benefits of GJA1-20k in a pig model of ischemia- reperfusion injury characterized by hemorrhage, prolonged aortic occlusion, and resuscitation. For this project, ATP concentration and interleukin-6 (IL6) RNA fold change were quantified from flash-frozen heart and renal cortex samples. Investigating tissue ATP concentration was a logical next step since GJA1-20k reduces oxygen consumption and regulates mitochondrial dynamics. IL6 transcription was evaluated since IL6 is a biomarker of injury severity in trauma patients and could be another indicator of GJA1- 20k overall protective mechanisms. It was found that with intravenous GJA1-20k treatment, the renal cortex had increased ATP concentrations but not the heart. GJA1-20k treatment had no significant effect on IL6 gene expression. These results further support GJA1-20k as a potential therapeutic drug for trauma-associated ischemia-reperfusion injury and give further insight into its mechanism of action.
INTRODUCTION
Uncontrolled bleeding is the primary cause of preventable death in trauma patients. Severe blood loss, or hemorrhagic shock, is responsible for 30 to 40% of mortality for trauma victims in the U.S. [1], [2], [3]. The pathophysiology of hemorrhagic shock is biphasic. First, patients suffer from ischemia, an inadequate delivery of oxygen to tissues resulting directly from blood losses. This leads to insufficient supplies for cellular metabolic needs. Ischemia in patients affected by hemorrhagic shock is commonly treated with blood transfusions, leading to reperfusion of oxygen and nutrients to cells in affected tissues [4], [5]. However, blood reperfusion during resuscitation circulates harmful metabolic byproducts that have built up systemically during the ischemic phase. Hemorrhagic shock is therefore characterized by ischemia-reperfusion injury (IRI), the paradoxical exacerbation of tissue damage caused by the reintroduction of oxygen to ischemic tissue [4], [6], [7]. IRI is characterized by oxidative stress, inflammation, apoptosis, and, importantly, mitochondrial dysfunction leading to cell death and ultimately multiorgan failure [3], [6]. Mitochondria are responsible for cellular energy production, metabolic regulation, and cell signaling [8]. During IRI, mitochondrial damage leads to reactive oxygen species (ROS) production, apoptosis, and energy depletion [4]. Mitochondrial dysfunction in IRI is linked to multiorgan failure and higher mortality rates [9]. Mitochondria play a vital role in IRI pathophysiology [8], [10]. Mitochondrial dysfunction is, therefore, a potential therapeutic target in trauma patients [6], [11]. The mitochondrial mechanisms contributing to IRI must be further investigated to identify appropriate treatment methods.
GJA1-20k, a peptide endogenous to mammals, protects the mitochondria during IRI [12], [13], [14]. IRI increases GJA1-20k expression such that GJA1-20k overexpression is a natural response to tissue injury [14]. GJA1-20k’s effects on mitochondria are reduced size from fission [14], relocation to the cell periphery [15], decreased respiration [16], and decreased ROS output [17]. The C-terminus tail of the GJA1-20k peptide interacts with the actin scaffolding around the mitochondria to cause mitochondrial fission [13], [17]. Interactions with microtubules and actin allow GJA1-20k to shuttle the smaller mitochondria to the cell periphery [15], [18], [19], which helps create a protective cytosolic mitochondrial dispersion. When GJA1-20k interacts with the mitochondria, it decreases mitochondrial respiration, ROS production, and mitochondrial membrane potential [12], [17]. Thus, less oxygen is consumed in an environment already deprived of oxygen, and metabolism is slowed. The protective effects of GJA1-20k are critical to the survival of tissues experiencing ischemia since mitochondria regulate cell apoptosis, and release damaging agents such as ROS and mitochondrial DNA [20].
The mechanisms behind the protective effects of GJA1-20k delivered as a drug are not fully understood. A preliminary study used a pig model of combined hemorrhagic shock and complete aortic occlusion to lead to profound IRI. Results from this preliminary study showed intravenous GJA1-20k reduced resuscitation fluid requirements [11] and decreased serum levels of interleukin 6 (IL6), which is a biomarker of injury severity in trauma patients [21], [22]. How the decreased oxygen consumption affects adenosine triphosphate (ATP) production is not understood. Quantifying ATP in tissue is essential for assessing metabolic failure and mitochondrial dysfunction. ATP serves as a key indicator of cellular energetics because its depletion reflects impaired oxidative phosphorylation due to reduced oxygen and nutrient delivery [20]. Given the central role of mitochondria in ATP production, its measurement provides insight into the extent of mitochondrial injury and the efficacy of therapeutic interventions targeting mitochondrial function. Furthermore, ATP quantification allows for the evaluation of tissue-specific susceptibility to ischemia and the metabolic impact of IRI following resuscitation. Given the physiological similarities between pigs and humans, these findings have important translational implications for improving resuscitation strategies in trauma patients.
Quantifying IL6 in tissue is important because it provides insight into localized inflammatory responses and the tissue-specific effects of GJA1-20k after hemorrhagic shock. While preliminary studies have demonstrated that GJA1-20k reduces serum IL6 levels [21], systemic cytokine concentrations do not always reflect tissue-specific inflammation. Tissue specific inflammation is important to investigate because it plays a critical role in organ dysfunction and injury. Measuring IL6 gene expression in key organs (such as the kidney and heart) allows for a more precise evaluation of how GJA1-20k modules inflammatory signaling at the tissue level. Determining IL6 gene expression clarifies whether GJA1-20k effects on IL6 are due to reduced systemic cytokine production, enhanced clearance, or direct mitigation of local inflammation. This distinction is crucial for understanding the mechanism of action of GJA1-20k and its potential role in protecting organ function after hemorrhagic shock.
This study hypothesizes that in a pig model of IRI, GJA1-20k therapy significantly increases ATP concentration and decreases IL6 gene expression in the heart and kidney. Controlled hemorrhage and aortic occlusion via resuscitative endovascular balloon occlusion of the aorta (REBOA) were used to model IRI. Pigs were treated intravenously with saline or GJA1-20k. The heart was studied because GJA1-20k is more concentrated in heart tissue [14]. The kidney was evaluated since acute kidney failure is a common morbidity of hemorrhagic shock [5], [23], and this model of IRI has been shown to have increased serum creatinine levels, a clinical biomarker of kidney injury [24], [25]. The flash-frozen heart and kidney tissues were evaluated for ATP concentration and IL6 gene expression. Investigating the mechanisms of GJA1-20k therapy enhances understanding of its mechanisms of action and advances its potential use in treating IRI from hemorrhagic shock.
BACKGROUND
Blood circulates oxygen and nutrients in the body. Hemorrhagic shock, or severe bleeding, is one of many conditions that may lead to reduced oxygen delivery to the cells. This is a pressing problem because of the high prevalence of hemorrhagic shock [26]. Not only is hemorrhage associated with high acute mortality rates, but those who survive the original injury often have lasting effects. Severe hemorrhage is also associated with elevated long-term mortality [26]. These patients have a shorter life expectancy, as well as other complications such as acute kidney injury [23], [27]. Even with treatment, patients who experience hemorrhage have increased morbidity and mortality rates compared to patients who have not experienced hemorrhage [2].
The kidneys are especially susceptible to damage during IRI. Animal studies consistently demonstrate elevation in serum creatinine after IRI [24], [28], which indicates kidney injury [25]. During severe hemorrhage blood flow to the kidney and other organs is reduced to conserve blood circulation to the heart and brain [29]. After ischemia, restoration of blood flow to the kidney can cause even more damage [29]. Acute kidney injury is considered an independent risk factor after trauma, and even mild acute kidney injury increases mortality risks in trauma patients by 2.5 times [23]. Furthermore, acute kidney injury in those patients can transition into chronic kidney disease, contributing to long-term morbidity [27]. Thus, the kidneys are especially susceptible to damage during IRI and require further protection in patients experiencing hemorrhagic shock.
Mitochondrial dysfunction is a hallmark of IRI because mitochondria need oxygen for some of their basic functions. The mitochondria are responsible for managing the amount of calcium in the cell, initiating cell apoptosis, and regulating metabolic processes [20]. Metabolic processes such as fatty acid synthesis, ATP production, amino acid metabolism, etc. are all regulated by the mitochondria [8]. The internal structure of the mitochondria (Figure 1) and the supply of oxygen is integral to mitochondrial function because of the electron transport chain (ETC).
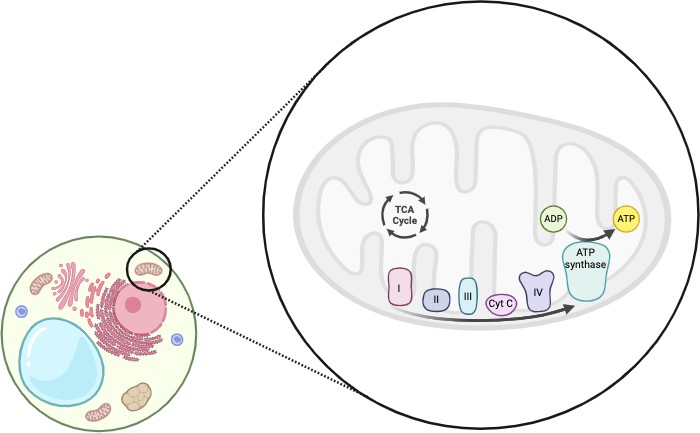
Figure 1. The mitochondria are an organelle inside of eukaryotic cells. Mitochondria have two membranes, the inner membrane and the outer membrane. In between the inner membranes of the mitochondria there are metabolic reactions such as the TCA cycle. Embedded in the inner membrane are the components of the electron transport chain, including complex I, II, III, IV, etc. This figure was adapted from [3] using Biorender.
Mitochondria use the reaction between oxygen and the electron transport chain to drive metabolic function. Oxygen is the ETC’s electron sink and drives electrons through its four complexes. Using this energy the ETC drives the creation of a proton gradient and, ultimately, the function of ATP synthase to produce ATP. Figure 2 shows how electrons travel along the inner mitochondrial membrane to pump protons into the intermembrane space. The electrons then reduce oxygen to form water, and the proton gradient is used to synthesize ATP. The proton gradient creates a membrane potential that the mitochondria also use to regulate other metabolic pathways. Thus, when tissues do not have enough oxygen during ischemia, mitochondria are some of the first organelles to stop functioning correctly. Oxygen depletion inhibits the ETC since there is no oxygen to accept the electrons. Instead, electrons react with other molecules to create damaging ROS.
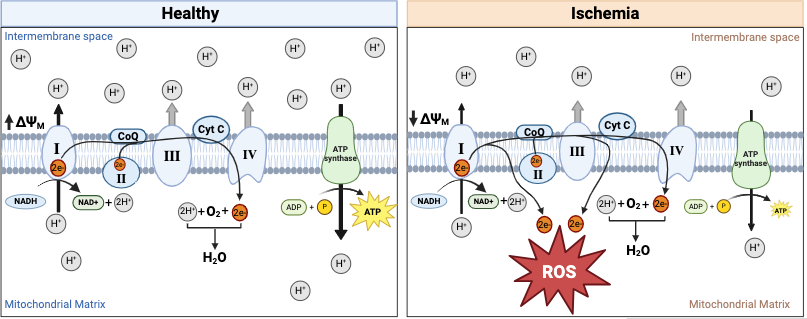
Figure 2. The ETC is integral to ATP production and metabolic regulation in the mitochondria [30]. The ETC is a series of protein complexes (I-IV) embedded in the inner mitochondrial membrane that drive ATP production through oxidative phosphorylation. Electrons from NADH and FADH2 are transferred through these complexes, generating a proton gradient across the membrane. Complex I and complex II transfer electrons to ubiquinone (Q), which carries them to complex III. Electrons are then passed to cytochrome c (Cyt C) and delivered to complex IV, where they reduce oxygen to water. The proton gradient created by electron transfer powers ATP synthase, driving the conversion of ADP to ATP. Without enough oxygen, the ETC will produce ROS, the membrane potential will decrease, and ATP production will decrease. During ischemia, complexes I and III of the ETC have increased ROS production as their electrons flow to other molecules instead of complex IV. This figure was adapted from [30] using Biorender.
The membrane potential also decreases duringischemia because the ETC no longer maintains the proton gradient. The decreased membrane potential reduces ATP production [6] and disables the mitochondria from regulating metabolism. This loss of membrane potential also disrupts mitochondrial homeostasis, leading to increased ROS production and the release of pro-apoptotic factors such as cytochrome c. Additionally, a diminished membrane potential compromises ion regulation, contributing to calcium overload and mitochondrial dysfunction.
ROS production is a hallmark of IRI owing to the reintroduction of oxygen after a period of hypoxia. ROS is an umbrella term for damaging agents such as free radicals, hydrogen peroxide, and reactive nitrogen species. ROS can damage DNA, proteins, lipids, and carbohydrates. As for mitochondrial ROS formation, the specific areas of the ETC where ROS can be formed are shown in Figure 2. It is normal for cells to have a small amount of ROS, but increased ROS levels are observed in tissues undergoing ischemia [26]. Increased levels of ROS come from an imbalance between ROS production and antioxidants. The accumulation of ROS damages the mitochondria and can lead to irreversible cell damage through the release of damage-associated molecular patterns (DAMPs) [26]. ROS production is associated with inflammation, necrosis, and apoptosis. This shows that ROS are directly related to a molecular pathway leading to cell death and inflammation.
Mitochondria can signal the cell to undergo apoptosis through small molecules called DAMPs when damaged by lack of oxygen [14]. Mitochondria can cause the cell to undergo apoptosis by releasing DAMPs during mitochondrial damage or stress [8]. Therefore, mitochondria are an attractive target for patients with IRI [24]. The peptide GJA1-20k protects the mitochondria during IRI and is a potential therapeutic drug for IRI.
GJA1-20k was first pinpointed as a protein of interest for the treatment of IRI when higher expression was found in cells subjected to oxidative stress [17], [31]. The GJA1 gene codes for Connexin 43 (Cx43), a gap junction protein essential for heart cells to communicate and synchronize function [17]. This large protein has many transmembrane regions [32]. The GJA1 gene also codes for GJA1-20k, a protein that is only 20 kilodaltons compared to Cx43 which is over twice that size [15], [33]. The GJA1 gene can be translated into a protein in a few different ways because there are several start codons, as shown in Figure 3 [17], [32]. One of the shorter protein versions is GJA1-20k, the most abundant isoform of Cx43 [15], [17]. GJA1-20k has the complete C-terminus tail and part of a transmembrane region of Cx43, as shown in Figure 3 [17]. GJA1-20k only has one transmembrane region and is not transported to the cell membrane in vesicles like the Cx43, meaning the tail end of the protein can interact with more components inside the cell than Cx43 [13]. Thus, GJA1-20k has an entirely different function from Cx43, even though they are both translated from the same gene.
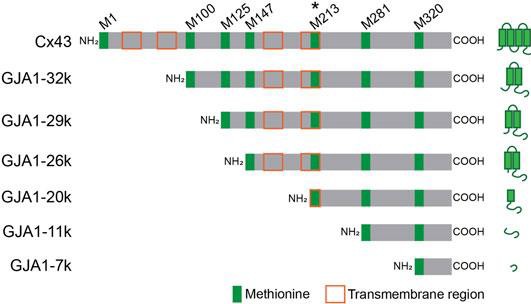
Figure 3. Cx43 has 6 common isoforms of which GJA1-20k is the most common [17]. GJA1-20k includes one transmembrane region and the C-terminus tail of Cx43.
GJA1-20k interacts with actin to stabilize it, and in keeping actin from breaking apart, it effectively controls the growth of actin filaments [13], [34]. During oxidative stress, GJA1-20k localizes to actin around the mitochondria’s outer membrane and controls the actin to change mitochondrial morphology [16]. GJA1-20k stabilizes actin filaments, directly coordinating mitochondrial fission [13]. Therefore, GJA1-20k causes fission of the mitochondria by directly interacting with actin instead of using a signaling cascade. It is not understood what function this GJA1-20k mediated fission is serving. However, the GJA1-20k mediated fission is related to GJA1-20k’s protection of the mitochondria after ischemic stress.
It is not entirely understood how GJA1-20k-mediated mitochondrial fission protects the mitochondria. However, there are a few known effects of GJA1-20k on mitochondria, even if the precise mechanism of mitochondrial protection is yet to be discovered. One study of GJA1-20k in heart cells under oxidative stress found that GJA1-20k caused metabolic hibernation and mitochondrial biogenesis [16]. Newly formed smaller mitochondria under the influence of GJA1-20k did not require as much oxygen or underwent metabolic hibernation. Therefore, GJA1-20k not only causes mitochondrial fission but also regulates the metabolic needs of the mitochondria. Another study found that GJA1-20k decreased mitochondrial maximal respiratory capacity [16]. In the presence of GJA1-20k, the mitochondria are less dependent on oxygen delivery. GJA1-20k is hypothesized to induce an adaptive response whereby mitochondria become more efficient in an environment deprived of oxygen. Therefore, it is hypothesized that ATP concentration would be higher in GJA1-20k-treated ischemic tissue.
Preliminary results showed that pigs treated with GJA1-20k after profound IRI needed less fluid injections to maintain their blood pressure after hemorrhage [11]. These pigs also displayed lower serum creatinine concentration at the end of the experiment [11]. Another set of preliminary results showed that serum concentrations of the protein IL6 were decreased from GJA1-20k therapy [21]. IL6 is a protein common in the bloodstream during ischemia [5]. It is primarily secreted by macrophages and other immune system cells and can act as a pro-inflammatory and anti-inflammatory cytokine [35]. The levels of IL6 in the serum are used as early biomarkers of injury severity [22]. Thus, GJA1-20k may increase IL6 secretion or gene expression. It is hypothesized therefore that the IL6 mRNA would be less abundant in the pigs who received a GJA1-20k injection.
GJA1-20k protects the mitochondria during ischemia and has potential therapeutic uses after IRI. The mechanisms of GJA1-20k must be further investigated to realize its therapeutic potential. So, in this study a pig model of hemorrhagic shock was used to investigate the effects of GJA1-20k treatment on ATP concentration and IL6 gene expression.
METHODS
Pig Surgery
All animal use protocols were approved by the University of Utah’s IACUC. Non-pregnant female or castrated male Yorkshire swine weighing between 50 and 75 kg were selected for the study. Pigs were used in this study to make results more translatable to human medicine [36]. All animals were acclimated to their enclosures for 10 days. Before anesthesia, the animals underwent an 8-12 hour fasting period, with free access to water.
Anesthesia was induced with an intramuscular injection of tiletamine/zolazepam at a dosage of 6-8 mg/kg. Endotracheal intubation was performed using a cuffed endotracheal tube to secure the airway. Following intubation, anesthesia was maintained with gaseous isoflurane at a concentration of 1.5-3.0% delivered in 2 L/min of oxygen. Mechanical ventilation was provided with a positive end- expiratory pressure set to 4 cmH₂O, and tidal volumes were maintained between 6-8 mL/kg. The respiratory rate was adjusted to achieve an end-tidal CO₂ of 35-45 mmHg. The fraction of inspired oxygen was regulated between 21% and 100% to maintain pulse oximetry readings between 95% and 98% throughout the procedure.
Warmed, balanced isotonic fluids (Plasmalyte 148) were administered intravenously at a 5 mL/kg/hour rate. For analgesia, continuous-rate infusion of hydromorphone was provided, starting with an initial dose of 0.025-0.05 mg/kg intravenously, followed by a continuous infusion of 0.03 mg/kg/hour IV for the duration of the study.
Animals were positioned in dorsal recumbency, and monitoring equipment, including electrocardiograms and temperature probes, was applied. To prevent hypothermia, animals were placed on warming blankets set to 39°C. Blood samples were collected for complete blood count and serum creatinine analysis. Animals with a plasma creatinine concentration exceeding 2.3 mg/dL were excluded from the study. Blood chemistry imbalances, including hypoglycemia, hyperkalemia, and ionized hypocalcemia, were corrected as needed throughout the experiment.
Before surgical preparation, the skin over each access site was cleaned using three alternating scrubs of chlorhexidine (or betadine) and alcohol. Following the third scrub, a final application of chlorhexidine or betadine was applied and allowed to dry completely. Once dry, each surgical site was draped in a sterile manner.
All vascular access sites were infiltrated with 2% lidocaine subcutaneously. Vascular catheters were placed under ultrasound guidance using the Seldinger technique, with open dissection performed if the percutaneous approach was unsuccessful. The following catheters were inserted (Figure 4):
- Two 7-9 Fr femoral arterial lines: one for continuous blood pressure monitoring and the other for blood sampling and REBOA catheter insertion (ER-REBOA, Prytime Medical, TX or a custom-made catheter).
- A 5 Fr carotid arterial line to monitor blood pressure above the occlusion point.
- A 9 Fr dual-lumen resuscitation line in the internal jugular vein for administering whole blood and fluid boluses, along with a pulmonary artery catheter placement to measure cardiac output and systemic vascular resistance.
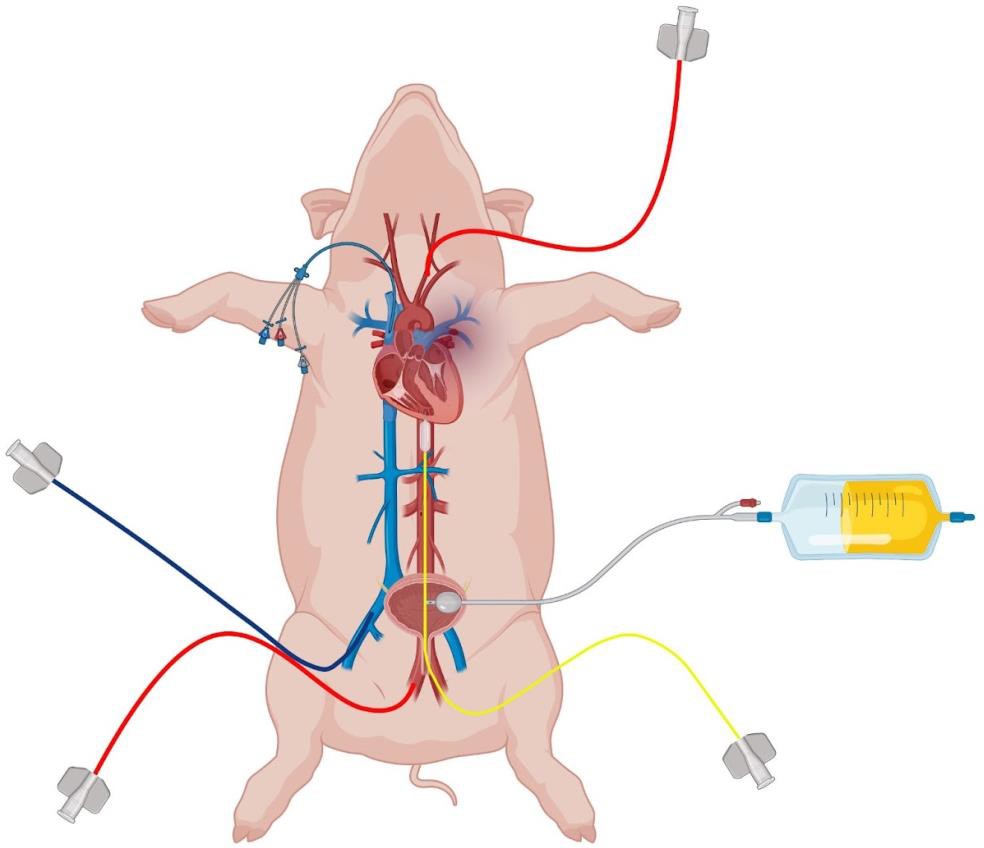
Figure 4. The pig was prepared for surgery by inserting a urinary catheter, two femoral arterial lines (one of which is shown in this figure), a carotid arterial line, two catheters in the internal jugular vein (one of which is shown in this figure), and a femoral vein catheter. These are used for blood draws, insertion of the REBOA catheter, blood pressure monitoring, etc. This figure was created using Biorender.
4. A triple-lumen catheter in the contralateral internal jugular vein to monitor central venous pressure placed in the opposite jugular vein to prevent artifactual elevation in central venous pressure during blood product administration.
5. A 7-9 Fr femoral vein catheter for administering crystalloid boluses during resuscitation.
A urinary catheter was placed via cystotomy, and a perivascular flow probe was positioned around the renal artery. A splenectomy was also performed [37]. The abdomen was closed until the conclusion of the experiment.
Animals were excluded if their plasma creatinine concentration was >2.3 mg/dL or their mean arterial pressure remained below 65 mmHg despite up to two 5 mL/kg boluses of isotonic crystalloids and isoflurane down-titration.
Figure 5 represents an overview of the experimental timeline. Following equilibration, 25% of each pig’s blood volume (estimated as 0.25 x 60 mL/kg x body weight in kg) was withdrawn over 30 minutes through a femoral arterial line, with the blood collected under constant agitation in citrated bags. After an additional 30 minutes, animals were subjected to 45 minutes of REBOA above the diaphragm (T60-T105), followed by a gradual balloon deflation over 10 minutes (T105-T115). The REBOA was placed in zone 1 (distal thoracic aorta).
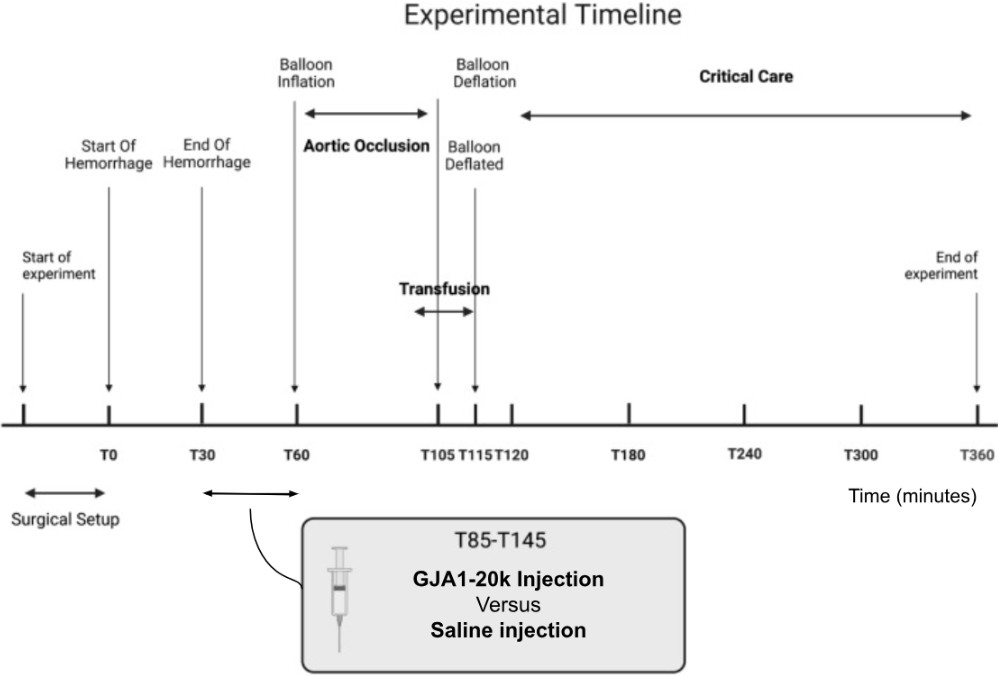
Figure 5. The general timeline of the pig surgery was adapted from another study [24]. This timeline shows the surgical setup, hemorrhage, drug administration, REBOA inflation/deflation, blood transfusion, and finally, euthanasia.
The LPS-free peptide used in this study (GJA1-20k) was obtained from Welgen Inc. (Worcester, MA), with quality and authenticity verified by the supplier and independently validated by our laboratory. GJA1-20k was then administered. The dose was chosen by first using the same dose of 0.1 mg/kg as another mitochondrial protectant [24]. This dose had a high mortality rate and was then decreased to 0.01 mg/kg dissolved in 60 mL of 0.9% saline. The peptide, with the same sequence as the human native peptide, was administered intravenously over 30 minutes from T30-T60. Control animals received an equivalent volume of 0.9% saline. Investigators were blinded to peptide or saline administration to minimize bias.
At the end of REBOA, pigs were reinfused with their own withdrawn blood over 10 minutes (T100-T110) and resuscitated with intravenous fluids (Plasmalyte 148, Baxter, IL) and vasopressors (norepinephrine, Hospira, IL) following a predefined protocol until T360. At T360 the pig was euthanized using a euthanasia solution overdose. The euthanasia was confirmed by monitoring for electrical activity and pulsatility of the arterial waveform.
Tissue from the left ventricle of the heart and cortex of the kidney were gathered and flash-frozen using liquid nitrogen. Heart tissue was collected because GJA1-20k expression is significantly increased in the heart after IRI [14]. Kidney tissue was collected because acute kidney failure is a common comorbidity of hemorrhagic shock [5], [23], and this model of IRI has been shown to have increased levels of serum creatinine, a marker of kidney injury [24], [25]. Tissues were stored at -80 °C without thawing and refreezing cycles [38]. The surgical procedure and tissue collection were repeated for 12 pigs, with six receiving GJA1-20k treatment and six receiving treatment with saline.
ATP Concentration
The ATP concentration was determined for heart and renal cortex tissue from pigs treated with GJA1-20k and those treated with saline. First, 100 +/- 10 mg of tissue was put in 500 uL of 1X Reaction Buffer from the ATP determination kit (Molecular Probes ATP determination kit A22066, Eugene, OR). This solution was chopped 30 times with scissors and then homogenized with a tissue rupture device for 10 seconds. Then the samples were spun at 15,000 rcf for 5 minutes at 3 °C. The supernatant was then removed, and the pellet was thrown away. The Pierce® BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA) and SpectraMax® M Series Multi-Mode Microplate Reader (SeqGen, Los Angeles, CA) were then used to determine the protein amount for each sample.
Next, the Molecular Probes® ATP Determination Kit A22066 was used to determine the amount of ATP in the samples. The instructions for the ATP determination kit were followed to create a reaction mixture. Next, 10 µL for the sample/standard and 90 µL of the reaction mixture were put in each well of a 96-well microplate. The plate was then put on a rocker for 30 seconds. The plate was read by a SpectraMax® M Series Multi-Mode Microplate Reader using luminescence mode with 500 integration. To calculate the ATP concentration, the ATP amount for each sample was divided by the protein amount for each sample.
IL6 Gene Expression
RT-qPCR was run on heart and renal cortex tissue from pigs treated with GJA1-20k and those treated with saline [39]. The RNA from the flash-frozen heart tissue and flash-frozen renal cortical tissue was isolated using the Qiagen RNeasy® Fibrous Tissue Mini Kit (Venlo, the Netherlands). The iScript™ Reverse Transcription Supermix for RT-qPCR (BioRad, Hercules, CA) was used along with the BIORAD Thermal Cycler (Hercules, CA) to convert the isolated RNA into cDNA. The instructions for iScript™ Reverse Transcription Supermix for RT-qPCR were followed for this conversion. GAPDH was the reference gene for heart and kidney RT-qPCR [40]. Primers for IL6 and GAPDH were designed by the University of Utah Health Science DNA/Peptide Synthesis Core (Table 1) [41].
The BIORAD CPX Opus Real-Time PCR System (Hercules, CA) determined the IL6 gene expression. The BIORAD SsoAdvanced Universal SYBR Green Supermix (Hercules, CA) instructions and products were used to run the RT-qPCR. The delta delta Cq method was used to determine the gene expression of IL6 normalized by GAPDH [42].
|
Table I Primers Used for RT-qPCR of IL6 and Housekeeping Gene GAPDH |
||
|
Gene Selected for |
Forward/Reverse |
Primer Sequence |
|
GAPDH |
Forward |
TTGGCTACAGCAACAGGGTG |
|
GAPDH |
Reverse |
GGGGAGATTCAGTGTGGTGG |
|
IL6 |
Forward |
GACAAAGCCACCACCCCTAA |
|
IL6 |
Reverse |
CTCGTTCTGTGACTGCAGCTTATC |
Statistical Analysis
The STATA statistics software was used for all the statistical analyses. For each data set normality was determined using the joint skewness and kurtosis test. The data were considered normal if the p-value for the joint skewness and kurtosis test was greater than or equal to 0.05. If the data were normal, the standard deviation and mean were calculated. The statistical significance was determined for normally distributed data using a two-sample t-test with equal variances [43]. If the data were not normal, the median and quartiles were calculated. For data that are not normally distributed the statistical significance was determined using a two-sample Wilcoxon rank-sum (Mann-Whitney) test [43]. Tests of statistical significance compared GJA1-20k to control groups in each data set. Significance was set at a p-value less than or equal to 0.05.
RESULTS
Twelve pigs underwent controlled hemorrhage and complete aortic occlusion to induce profound IRI. The pigs were treated intravenously with GJA1-20k or saline as a control. Tissue ATP concentration and IL6 gene expression were then determined for the left ventricular heart and the renal cortex.
ATP Concentration
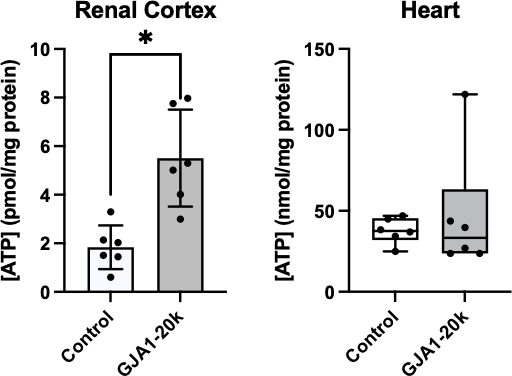
Figure 6. Concentration of ATP was compared between GJA1-20k and control groups in the renal cortex and the heart. A) Since values for the renal cortex were normally distributed, mean and standard deviation are represented. On average, the renal tissue ATP concentration was 3.7 pmol/mg protein higher in the GJA1-20k treatment group than control. B) Since values for the heart were not normally distributed, median, interquartile range, maximums, and minimums are represented. The asterisk indicates a significant difference between the control and GJA1-20k treatment in the ATP concentration of the renal cortex. All black dots represent individual ATP concentration values. (* p= 0.0021)
ATP concentration in the renal cortex was significantly higher with GJA1-20k treatment (p = 0.0021) (Figure 6). ATP concentration in the renal cortex was found to be normally distributed (p = 0.43), while the ATP concentration assay in the heart was not normally distributed (p= 0.0002). Statistical analysis revealed that there was no significant difference between the GJA1-20k treatment and control group in myocardial ATP concentration (p = 0.63).
IL6 Gene Expression
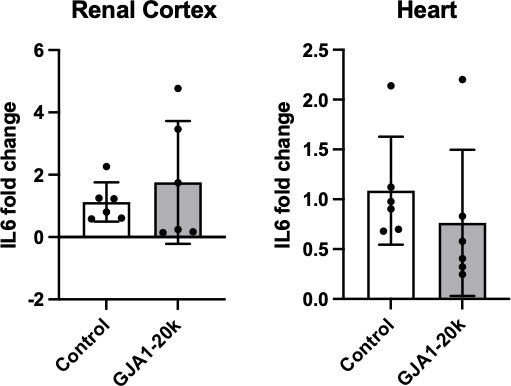
Figure 7. Fold change of IL6 mRNA gene expression in the renal cortex and the heart. A. Since values for the renal cortex were normally distributed, mean and standard deviation are represented. B. Since values for the heart were also normally distributed, mean and standard deviation are represented. All black dots represent individual IL6 fold change values.
No significant difference in IL6 gene expression was found between control and GJA1-20k treatment in the renal cortex (p = 0.47) or the heart (p = 0.40) (Figure 7). The fold change of IL6 gene expression in the renal cortex was found to be normally distributed (p = 0.053). The fold change in IL6 gene expression in the heart was normally distributed (p = 0.084).
DISSCUSION
Hemorrhagic shock, or severe blood loss, causes 1.9 million deaths every year through ischemia, or a lack of oxygen in the tissues [2]. Ischemia-reperfusion injury (IRI) exacerbates tissue damage caused by reinfusing ischemic tissues with blood and oxygen [4]. GJA1-20k, a stress response protein, is vital to tissue survival during ischemia because it protects the mitochondria which can regulate cell apoptosis [16]. This study hypothesized that intravenous injection of GJA1-20k increases ATP concentration and decreases interleukin 6 (IL6) gene expression in the heart and kidney in a pig model of profound IRI. Twelve pigs underwent hemorrhage and aortic occlusion and were then treated intravenously with either saline as a control or the protein GJA1-20k. Heart and renal cortex tissue were dissected and analyzed for IL6 gene expression and ATP concentration. Comparisons of IL6 and ATP changes versus saline controls were used to assess the impact of GJA1-20 therapeutic intervention.
Figure 6 indicates that GJA1-20k therapy significantly increased ATP concentration in the renal cortex but not the heart after IRI. The increased ATP concentration could be due to healthier mitochondria or direct effects on metabolic pathways. During ischemia, GJA1-20k reduces oxygen consumption [16], supposedly reducing ATP production. However, the already-listed protective effects of GJA1-20k could cause healthier mitochondria that would produce more ATP. GJA1-20k as a membrane protein could also increase ATP production in the mitochondria more directly.
GJA1-20k has a different effect on ATP concentration in the heart vs. the kidney tissue. This may be due to a difference in tissue localization of GJA1-20k or reactions happening within the tissue. The kidneys may express a unique or more abundant receptor for GJA1-20k that is absent or less concentrated in the heart. A receptor difference could drive the peptide to localize to the kidneys preferentially. Resuscitative endovascular occlusion of the aorta (REBOA) inserted into zone 1 causes more severe kidney ischemia than the heart [44]. Also, blood circulation is naturally conserved during severe hemorrhage to preserve the heart and brain, not the kidney [29]. As a result, GJA1-20k may localize to and exert a more significant effect on areas experiencing more severe ischemia. Conversely, GJA1-20k is endogenously expressed more in the heart [14], so the intravenous GJA1-20k may have an increased effect on the kidney because there is less endogenous expression. Multiple theories can be proposed for this difference in GJA1-20k effect based on the tissue, but further investigation is required to determine causation.
IL6 gene expression did not significantly increase, as shown in Figure 7. The decrease in IL6 in the serum of the pigs who underwent GJA1-20k therapy [21] is probably from reduced secretion, either from the tissue, inflammatory cells, or both. IL6 secretion is essential to the immune system’s inflammatory response and is commonly secreted by immune cells like macrophages [35]. Thus, GJA1- 20k may be interacting with the cells secreting IL6.
In contrast to other studies of GJA1-20k, this is the first time that GJA1-20k has been injected intravenously instead of translated within the cells. Other studies have been conducted in GJA1-20k knockout and knockdown mice [17], GJA1-20k endogenous gene expression [16], or the effect of increased GJA1-20k expression introduced through a virus [15]. However, the ability of GJA1-20k to have a positive effect through a simple injection makes it more viable as a drug in a clinical setting. In this study, published preliminary data showed that the pigs treated with GJA1-20k as an intravenous injection did not require as many fluid injections to maintain a normal blood pressure after IRI [11]. Other published preliminary data showed that these pigs treated with GJA1-20k injections after ischemia had lowered serum IL6 levels [21]. These pigs had significantly increased ATP concentration in the kidney from GJA1-20k therapy, as shown in Figure 6. Thus, further effects of GJA1-20k therapy should be investigated since several significant effects have already been found.
Other drugs are also being investigated as to their effects on the mitochondria during IRI. The mitochondrial-targeted antioxidant SKQ1 reduces ROS, mitochondrial DNA, and inflammation in the heart of hemorrhaging rats [26]. Similarly, elamipretide protects the mitochondria in a pig model of IRI and when used as a drug, reduces resuscitative requirements and serum creatinine levels [24]. These other drugs are being investigated using similar methods including controlled hemorrhage, aortic occlusion, and drug administration during resuscitation.
Factors outside of the GJA1-20k therapy could have affected the ATP concentration in this study. The pigs who received GJA1-20k treatment consistently required less resuscitation fluids during the critical care phase of the surgery [11]. Other potential confounding variables in the pigs include gender, slight differences in age, and individual genetic differences. Increased sample size could confirm that the results were not skewed by some of these confounding variables.
Several factors could have contributed to the nonsignificant results found in IL6 gene expression, as seen in Figure 7. The euthanasia and longer cryostorage time could have reduced the ability to detect differences in IL6 gene expression. Tissue storage increases mRNA degradation, which could be prevented by performing this analysis quickly. In addition, six hours between starting hemorrhage and euthanasia may not give tissues enough time for IL6 to change gene expression. Usually, IL6 is secreted from macrophages and other immune system cells on a short time scale [35]. Six hours would not be enough time to investigate the long-term effects of the GJA1-20k injection. This would help explain why no significant difference was detected in IL6 gene expression as shown in Figure 7.
This study measured ATP concentration in the tissue, not the mitochondria. Increased ATP production is assumed to be from the mitochondria because GJA1-20k localizes to the mitochondrial membrane [16]. However, it is unknown whether the increase in ATP concentration is coming from oxidative phosphorylation or other metabolic pathways in the cytosol. In the future, the mitochondria could be isolated, and then the ATP concentration could be determined. This would help determine if the increased ATP production is happening because of changes to glycolysis in the cytosol or oxidative phosphorylation in the mitochondria. A respirometer could be used to analyze GJA1-20k effects on oxidative phosphorylation in the kidney. This would clarify how GJA1-20k affects each electron transport chain complex in the mitochondria. These studies would provide further insights into the mechanism of GJA1-20k in ATP production.
More work should also be done to understand where GJA1-20k localizes and how it moves throughout the body. Fluorescent tagging of dosed GJA1-20k and its analysis in collected tissues could be used to analyze cellular and organ-specific localization of GJA1-20k following intravenous injections during ischemia. The localization of GJA1-20k could also be tracked in real-time using a combination of radioactive tagging of GJA1-20k and magnetic resonance imaging (MRI) [45], [46]. GJA1-20k’s transmembrane region prevents it from diffusing across the cell membrane [17]. Therefore, when GJA1- 20k is injected, it is unknown whether GJA1-20k is crossing the cell membranes to affect the mitochondria [20]. If GJA1-20k is crossing the cell membranes, the mechanism by which it is doing so is unclear. The mechanism by which GJA1-20k enters the cells from the bloodstream could be investigated by looking at potential GJA1-20k cell receptors.
This study stopped only a few hours after GJA1-20k was injected and therefore investigated primarily the short-term impacts of GJA1-20k. Thus, studies should be done on the long-term effects of GJA1-20k to investigate its potential toxic effects as well as the rates of acute and chronic kidney injury after IRI [28]. Also, this study investigated the administration of GJA1-20k after the patient had already experienced ischemia. Future studies could examine if GJA1-20k could be used to prevent mitochondrial damage by giving the drug before ischemia is induced. The above-mentioned studies would be critical to GJA1-20k becoming a clinically relevant therapeutic drug.
GJA1-20k is critical because of its potential as a therapeutic drug. Patients with severe IRI could greatly benefit from pharmacological interventions that target the mitochondria [24]. Protecting the mitochondria could be especially relevant to reducing IRI associated with hemorrhagic shock. GJA1- 20k has now been shown to increase ATP in the kidneys when given intravenously, which could directly lead to less cell death after IRI [5]. ATP is a common output of mitochondria, and GJA1-20k has been shown to localize to and protect the mitochondria after IRI. Thus, GJA1-20k is a good potential therapeutic drug for mitochondrial dysfunction after IRI.
Acute kidney injury is a common side effect of IRI [27]. GJA1-20k protects the mitochondria after IRI and has significantly impacted the kidneys. GJA1-20k could help protect the mitochondria in the kidneys and prevent acute kidney injury after IRI. Not only could GJA1-20k be used as a treatment for hemorrhagic shock, but it could also treat other forms of ischemia. Surgeries with frequent IRI include solid organ transplants [6] and cardiopulmonary bypass surgeries [47]. GJA1-20k could be given to patients already experiencing ischemia to help their cells better manage and recover from oxidative stress. Therefore, GJA1-20k could be of interest as a therapeutic drug to healthcare workers involved in both emergency medicine and surgery to prevent complications from ischemia. This study identified one potential drug delivery technique for GJA1-20k. Therefore, GJA1-20k is a potential treatment for IRI in hemorrhagic shock and a variety of other clinical settings.
References
- M. El Sayad and H. Noureddine, “Recent Advances of Hemorrhage Management in Severe Trauma,” Emerg. Med. Int., vol. 2014, p. 638956, 2014, doi: 10.1155/2014/638956.
- J. W. Cannon, “Hemorrhagic Shock,” N. Engl. J. Med., vol. 378, no. 4, pp. 370–379, Jan. 2018, doi: 10.1056/NEJMra1705649.
- N. V. Andrianova, M. I. Buyan, A. A. Brezgunova, K. S. Cherkesova, D. B. Zorov, and E. Y. Plotnikov, “Hemorrhagic Shock and Mitochondria: Pathophysiology and Therapeutic Approaches,” Int. J. Mol. Sci., vol. 26, no. 5, Art. no. 5, Jan. 2025, doi: 10.3390/ijms26051843.
- M. Zhang, Q. Liu, H. Meng, H. Duan, X. Liu, J. Wu, F. Gao, S. Wang, R. Tan, and J. Yuan,
“Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets,” Signal Transduct. Target. Ther., vol. 9, no. 1, pp. 1–39, Jan. 2024, doi: 10.1038/s41392-023-01688-x. - M. Malek and M. Nematbakhsh, “Renal ischemia/reperfusion injury; from pathophysiology to treatment,” J. Ren. Inj. Prev., vol. 4, no. 2, pp. 20–27, Jun. 2015, doi: 10.12861/jrip.2015.06.
- R. O. S. Soares, D. M. Losada, M. C. Jordani, P. Évora, and O. Castro-e-Silva, “Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies,” Int. J. Mol. Sci., vol. 20, no. 20, Art. no. 20, Jan. 2019, doi: 10.3390/ijms20205034.
- P. Cowled and R. Fitridge, “Pathophysiology of Reperfusion Injury,” in Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, R. Fitridge and M. Thompson, Eds., Adelaide (AU): University of Adelaide Press, 2011. Accessed: Dec. 09, 2024. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK534267/
- E. Vringer and S. W. G. Tait, “Mitochondria and cell death-associated inflammation,” Cell Death Differ., vol. 30, no. 2, Art. no. 2, Feb. 2023, doi: 10.1038/s41418-022-01094-w.
- A. V. Kozlov, S. Bahrami, E. Calzia, P . Dungel, L. Gille, A. V. Kuznetsov, and J. Troppmair, “Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure?,” Ann. Intensive Care, vol. 1, p. 41, Sep. 2011, doi: 10.1186/2110-5820- 1-41.
- Q. Sun, W. Gao, P . Loughran, R. Shapiro, J. Fan, T. Billiar, and M. J. Scott, “Caspase 1 activation is protective against hepatocyte cell death by up-regulating beclin 1 protein and mitochondrial autophagy in the setting of redox stress,” J. Biol. Chem., vol. 288, no. 22, pp. 15947–15958, May 2013, doi: 10.1074/jbc.M112.426791.
- N. Ewer, R. Ford, V. Ngyen, D. Shimura, L. Neff, T. Williams, S. Youngquist, M. A. Johnson, R. Shaw, and G. Hoareau, “43: GJA1-20K REDUCES RESUSCITATION REQUIREMENTS AND RENAL INJURY POST-REBOA ISCHEMIA-REPERFUSION INJURY,” Crit. Care Med., vol. 51, no. 1, p. 22, Jan. 2023, doi: 10.1097/01.ccm.0000906048.66822.f6.
- “Stress response protein GJA1-20k promotes mitochondrial biogenesis, metabolic quiescence, and cardioprotection against ischemia/reperfusion injury – PMC.” Accessed: Oct. 24, 2023. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6237442/
- R. Baum, J. A. Palatinus, M. Waghalter, D. Shimura, Q. Jin, L. Kuzmanovich, S. Xiao, A. G. Kléber, E. E. Grintsevich, T. Hong, and R. M. Shaw, “GJA1-20k, an internally translated isoform of Connexin 43, is an actin capping protein,” Jan. 05, 2022, bioRxiv. doi: 10.1101/2022.01.05.475034.
- D. Shimura, E. Neubel, R. Baum, S. E. Valdez, S. Xiao, J. S. Warren, J. A. Palantinus, T. Hong, J. Rutter, and R. M. Shaw, “Protective mitochondrial fission induced by stress-responsive protein GJA1- 20k,” eLife, vol. 10, p. e69207, Oct. 2021, doi: 10.7554/eLife.69207.
- D. Ren, P . Zheng, J. Feng, Y. Gong, Y. Wang, J. Duan, L. Zhao, J. Deng, H. Chen, S. Zou, T. Hong, and W. Chen, “Overexpression of Astrocytes-Specific GJA1-20k Enhances the Viability and Recovery of the Neurons in a Rat Model of Traumatic Brain Injury,” ACS Chem. Neurosci., vol. 11, no. 11, pp. 1643–1650, Jun. 2020, doi: 10.1021/acschemneuro.0c00142.
- W. A. Basheer, Y. Fu, D. Shimura, S. Xiao, S. Agvanian, D. M. Hernandez, R. C. Hitzeman, T. Hong, and R. M. Shaw, “Stress response protein GJA1-20k promotes mitochondrial biogenesis, metabolic quiescence, and cardioprotection against ischemia/reperfusion injury,” JCI Insight, vol. 3, no. 20, p. e121900, Oct. 2018, doi: 10.1172/jci.insight.121900.
- D. Shimura and R. M. Shaw, “GJA1-20k and Mitochondrial Dynamics,” Front. Physiol., vol. 13, 2022, Accessed: Oct. 24, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphys.2022.867358
- Y. Fu, L. Tao, F. Peng, N. Zheng, Q. Lin, S. Cai, and Q. Wang, “GJA1-20k attenuates Ang II-induced pathological cardiac hypertrophy by regulating gap junction formation and mitochondrial function,” Acta Pharmacol. Sin., vol. 42, no. 4, pp. 536–549, Apr. 2021, doi: 10.1038/s41401-020-0459-6.
- Y. Fu, S. Zhang, S. Xiao, W. Basheer, R. Baum, I. Epifantseva, T. Hong, and R. M. Shaw, “Cx43 Isoform GJA1-20k Promotes Microtubule Dependent Mitochondrial Transport,” Front. Physiol., vol. 8, Nov. 2017, doi: 10.3389/fphys.2017.00905.
- D. C. Chan, “Mitochondria: Dynamic Organelles in Disease, Aging, and Development,” Cell, vol. 125, no. 7, pp. 1241–1252, Jun. 2006, doi: 10.1016/j.cell.2006.06.010.
- T. Hunt-Smith, R. Johnson, J. Themen, W. Mohamed, G. Lacey-Howard, S. Youngquist, A. Johnson, R. Shaw, and G. Hoareau, “1606: GJA1-20K REDUCES LEVELS OF INTERLEUKIN-6 IN PATIENTS WITH REBOA-INDUCED ISCHEMIA-REPERFUSION INJURY,” Crit. Care Med., vol. 52, no. 1, p. S786, Jan. 2024, doi: 10.1097/01.ccm.0001004580.65550.b5.
- P. K. Okeny, P. Ongom, and O. Kituuka, “Serum interleukin-6 level as an early marker of injury severity in trauma patients in an urban low-income setting: a cross-sectional study,” BMC Emerg. Med., vol. 15, no. 1, p. 22, Sep. 2015, doi: 10.1186/s12873-015-0048-z.
- B. E. Biesterveld, A. Z. Siddiqui, R. L. O’Connell, H. Remmer, A. M. Williams, A. Shamshad, W.M. Smith, M. T. Kemp, G. K. Wakam, and H. B. Alam, “Valproic Acid Protects Against Acute Kidney Injury in Hemorrhage and Trauma,” J. Surg. Res., vol. 266, pp. 222–229, Oct. 2021, doi: 10.1016/j.jss.2021.04.014.
- N. Patel, M. A. Johnson, N. Vapniarsky, M. W. Van Brocklin, T. K. Williams, S. T. Youngquist, R. Ford, N. Ewer, L. P . Neff, and G. L. Hoareau, “Elamipretide mitigates ischemia-reperfusion injury in a swine model of hemorrhagic shock,” Sci. Rep., vol. 13, no. 1, Art. no. 1, Mar. 2023, doi: 10.1038/s41598-023- 31374-5.
- H. Shahbaz, P. Rout, and M. Gupta, “Creatinine Clearance,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2025. Accessed: Feb. 08, 2025. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK544228/
- B. Jia, J. Ye, L. Gan, R. Li, M. Zhang, D. Sun, L. Weng, Y. Xiong, J. Xu, P . Zhang, W. Huang, M. Zheng, and T. Wang, “Mitochondrial antioxidant SkQ1 decreases inflammation following hemorrhagic shock by protecting myocardial mitochondria,” Front. Physiol., vol. 13, 2022, doi: 10.3389/fphys.2022.1047909.
- Y. Ishimoto and R. Inagi, “Mitochondria: a therapeutic target in acute kidney injury,” Nephrol. Dial. Transplant., vol. 31, no. 7, pp. 1062–1069, Jul. 2016, doi: 10.1093/ndt/gfv317.
- N. Pabla and A. Bajwa, “Role of Mitochondrial Therapy for Ischemic-Reperfusion Injury and Acute Kidney Injury,” Nephron, vol. 146, no. 3, pp. 253–258, Dec. 2021, doi: 10.1159/000520698.
- N. Fage, P. Asfar, P. Radermacher, and J. Demiselle, “Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics,” Int. J. Mol. Sci., vol. 24, no. 4, Art. no. 4, Jan. 2023, doi: 10.3390/ijms24044103.
- “Assays for ROS and oxidative stress : 주요브랜드 자세한 product information.” Accessed: Nov. 11, 2023. [Online]. Available: https://dawinbio.com/product- information/?q=YToyOntzOjQ6InBhZ2UiO2k6MjtzOjEyOiJrZXl3b3JkX3R5cGUiO3M6Mzo iYWxsIjt9&bmode=view&idx=16738139&t=board&category=8I0n7X1B5h
- C. C. Whisenant and R. M. Shaw, “Internal translation of Gja1 (Connexin43) to produce GJA1- 20k: Implications for arrhythmia and ischemic-preconditioning,” Front. Physiol., vol. 13, Dec. 2022, doi: 10.3389/fphys.2022.1058954.
- S. Xiao, D. Shimura, R. Baum, D. M. Hernandez, S. Agvanian, Y. Nagaoka, M. Katsumata, P. D.Lampe, A. G. Kleber, T. Hong, and R. M. Shaw, “Auxiliary trafficking subunit GJA1-20k protects connexin-43 from degradation and limits ventricular arrhythmias,” J. Clin. Invest., vol. 130, no. 9, pp. 4858–4870, Sep. 2020, doi: 10.1172/JCI134682.
- “Connexin 43/GJA1 Antibody,” www.rndsystems.com. Accessed: Nov. 09, 2023. [Online]. Available: https://www.rndsystems.com/products/connexin-43-gja1-antibody_pps045
- W. A. Basheer, S. Xiao, I. Epifantseva, Y. Fu, A. G. Kleber, T. Hong, and R. M. Shaw, “GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs,” Circ. Res., vol. 121, no. 9, pp. 1069–1080, Oct. 2017, doi: 10.1161/CIRCRESAHA.117.311955.
- M. Aliyu, F. T. Zohora, A. U. Anka, K. Ali, S. Maleknia, M. Saffarioun, and G. Azizi, “Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach,” Int. Immunopharmacol., vol. 111, p. 109130, Oct. 2022, doi: 10.1016/j.intimp.2022.109130.
- J. K. Lunney, A. Van Goor, K. E. Walker, T. Hailstock, J. Franklin, and C. Dai, “Importance of the pig as a human biomedical model,” Sci. Transl. Med., vol. 13, no. 621, p. eabd5758, Nov. 2021, doi: 10.1126/scitranslmed.abd5758.
- V. S. Bebarta, M. Daheshia, and J. D. Ross, “The significance of splenectomy in experimental swine models of controlled hemorrhagic shock,” J. Trauma Acute Care Surg., vol. 75, no. 5, p. 920, Nov. 2013, doi: 10.1097/TA.0b013e3182a539b8.
- J. Botling, K. Edlund, U. Segersten, S. Tahmasebpoor, M. Engström, M. Sundström, P .
Malmström, and P . Micke, “Impact of Thawing on RNA Integrity and Gene Expression Analysis in Fresh Frozen Tissue,” Diagn. Mol. Pathol., vol. 18, no. 1, p. 44, Mar. 2009, doi: 10.1097/PDM.0b013e3181857e92. - R. Harshitha and D. R. Arunraj, “Real-time quantitative PCR: A tool for absolute and relative quantification,” Biochem. Mol. Biol. Educ., vol. 49, no. 5, pp. 800–812, 2021, doi: 10.1002/bmb.21552.
- P. Arlock, M. Li, B. Davis. C. Lövdahl, Q. Liao, T. Sjöberg, A. Rahman, B. Wohlfart, S. Steen, and A. Arner, “Excitation and contraction of cardiac muscle and coronary arteries of brain‐ dead pigs,” FASEB BioAdvances, vol. 5, no. 2, p. 71, Dec. 2022, doi: 10.1096/fba.2022-00104.
- K. H. Ho and A. Patrizi, “Assessment of common housekeeping genes as reference for gene expression studies using RT-qPCR in mouse choroid plexus,” Sci. Rep., vol. 11, no. 1, p. 3278, Feb. 2021, doi: 10.1038/s41598-021-82800-5.
- D. A. Khan and S. Fatima, “B-218 Role of Delta Tocotrienol and Resveratrol Supplementation in Regulation of Micro RNAs in Patients With Metabolic Syndrome: A Randomized Controlled Trial,” Clin. Chem., vol. 69, no. Supplement_1, p. hvad097.545, Oct. 2023, doi: 10.1093/clinchem/hvad097.545.
- R. M. West, “Best practice in statistics: Use the Welch t-test when testing the difference between two groups,” Ann. Clin. Biochem., vol. 58, no. 4, pp. 267–269, Jul. 2021, doi: 10.1177/0004563221992088.
- S. Halvachizadeh, L. Mica, Y. Kalbas, M. Lipiski, M. Canic, M. Teuben, N. Cesarovic, Z.
Rancic, P . Cinelli, V. Neuhaus, H. Pape, and R. Pfeifer, “Zone-dependent acute circulatory changes in abdominal organs and extremities after resuscitative balloon occlusion of the aorta (REBOA): an experimental model,” Eur. J. Med. Res., vol. 26, no. 1, p. 10, Jan. 2021, doi: 10.1186/s40001-021-00485-y. - M. R. Edelmann, “Radiolabelling small and biomolecules for tracking and monitoring,” RSC Adv., vol. 12, no. 50, pp. 32383–32400, 2022, doi: 10.1039/D2RA06236D.
- A. J. Fischman, N. M. Alpert, and R. H. Rubin, “Pharmacokinetic Imaging,” Clin. Pharmacokinet., vol. 41, no. 8, pp. 581–602, Jul. 2002, doi: 10.2165/00003088-200241080-00003.
- R. C. Smith, J. M. Leung, and D. T. Mangano, “Postoperative myocardial ischemia in patients undergoing coronary artery bypass graft surgery. S.P.I. Research Group,” Anesthesiology, vol. 74, no. 3, pp. 464–473, Mar. 1991, doi: 10.1097/00000542-199103000-00013.

