Spencer Fox Eccles School of Medicine
69 The BASIC Trial: An ESKAPE from Transmission
Alayna Stoddard; Harriet Hopf; Tommy Brower; and Seth Evans
Faculty Mentor: Harriet Hopf (Anesthesiology, University of Utah)
Abstract
Since being given their own acronym in 2008, the ESKAPE pathogens— Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. have continued to become increasingly resistant to antibiotic therapies. With only a few new antibiotics being discovered in the 21st century, and the propensity of pathogens to develop resistance to new antibiotics, it’s imperative that we get back to the basics of infection control to reduce the reliance on antibiotics. These basics, hand hygiene, handling of the intravascular (IV) catheter and IV tubing, environmental cleaning, and patient decolonization, have been shown to reduce surgical site infection, yet uptake is far from universal. The BASIC Study is a 2×2 factorial randomized trial design that aims to understand the barriers to implementing these basic infection prevention protocols. The experimental implementation strategies are informed by pathogen transmission data that is collected in the operating room, allowing the healthcare team to see exactly how and where these pathogens are spread. The hypothesis of this study is that an evidence-based infection protocol (EBIP) informed by transmission data and implemented with coaching support will reduce perioperative ESKAPE pathogen transmission events by 40%.
Introduction
Reducing the incidence of surgical site infections (SSIs) and healthcare-acquired infections (HAIs) is no small challenge for the healthcare team. These infections cause significant harm to patients and impose a substantial financial burden on healthcare systems. At the root of these infections are ESKAPE pathogens—a group of bacteria responsible for the majority of nosocomial infections (Haque, et. al.). These pathogens are not only highly resilient but are also becoming increasingly resistant to antibiotic treatments, exacerbating the risks they pose to patient safety (Rice).
HAIs, particularly SSIs, are especially dangerous for individuals with compromised immune systems, with SSIs accounting for approximately 20% of all HAIs (Shepard). Of the 310 million surgeries performed annually (Dobson), 3-5% result in SSIs, equating to roughly 12.4 million cases per year. These infections double a patient’s risk of mortality (Brown, Loftus) and contribute to longer hospital stays, higher costs, and poorer outcomes overall (Zimlichman).
The growing prevalence and antibiotic resistance of ESKAPE pathogens highlights an urgent need for effective prevention and control strategies. These microbes thrive in hospital environments, where they exploit high-touch surfaces and healthcare interactions to spread. To mitigate their impact, it is critical to understand the specific pathways of transmission and implement targeted interventions. Protecting patients from these infections is a key to improving healthcare outcomes and reducing systemic costs.
The BASIC Study is an investigation into implementation strategies of evidence-based practices that can overcome the barriers that prevent the adoption of practices that decrease the transmission of the ESKAPE pathogens. These practices, often referred to as “The 4 Pillars,” include hand hygiene, intravascular catheter handling, environmental cleaning, and patient decolonization. Each one is designed to decrease pathogen transmission in the operating room, thereby protecting the patient from dangerous surgical site infections.
Methods
The BASIC Trial is an ongoing multicenter, NIH-funded, Institutional Review Board approved clinical trial that assesses the effectiveness of two implementation strategies: Technical Assistance (TA; control) and Evidence- BasedInfectionProtocol (EBIP/coaching). In this 2X2 randomized, factorial study, sites are randomized to EBIP with or without surveillance, surveillance alone, or control (TA). Technical Assistance involves monthly video calls that assess adherence to study protocol, while Evidence-Based Infection Protocol involves monthly team-based coaching sessions to help the anesthesia team work together to implement the infection prevention practices.
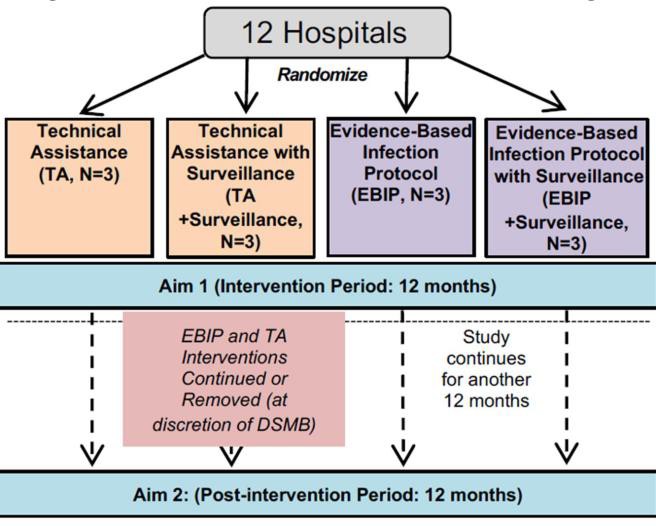
Figure 1. 2×2 Factorial Randomized Trial Design. The University of Utah is assigned to the TA without assistance arm.
Surveillance provides the bacterial presence and transmission data from the swabs collected from the operating room. This feedback loop allows targeted adjustment of the infection control strategy. The hypothesis is that the combination of EBIP and surveillance will be most effective in reducing cross-transmission in the operating room and will reduce transmission events by at least 40%. The University of Utah site was randomized to the TA/no surveillance arm (control/control arm). Therefore, we do not receive EBIP, nor do we know what is involved for sites that do, and we do not receive surveillance data. Patient recruitment is done in the University of Utah Orthopaedic Center Spine Clinic. Inclusion and exclusion criteria are outlined in Table 1.
Eligible Case 1 patients are identified and consented in the spine clinic. After consent is obtained, baseline swabs are collected in the clinic at least 10 days before surgery to ensure an accurate sample is collected. Case 2 patients are identified and approached for consent in the pre- op area on the day of surgery. Case 2 patients must be identified on the day of surgery because the operating room (OR) schedule is constantly changing, even up to the morning of surgery.
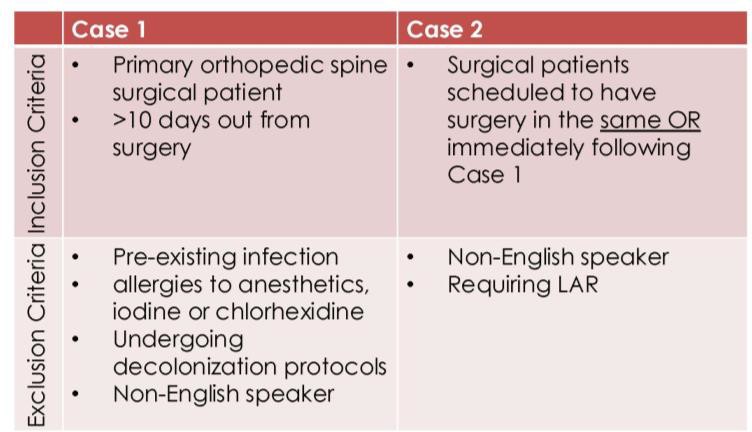
Table 1: Inclusion and exclusion criteria for Case 1 and Case 2 patients
Once a Case 1 patient is consented to the study, research staff collect a baseline sample in the clinic. This baseline sample must be collected at least 10 days before surgery to ensure the patient hasn’t started any form of decontamination protocol (e.g., nasal iodine, chlorhexidine wash, etc.) begins to ensure we are collecting an accurate baseline. We then follow these patients to surgery, where we collect pre-incision swabs from the patient’s groin, axilla and nose, OR, anesthesia provider(s), and the surgeon. These same swabs are repeated after the surgery closes. Further swabbing occurs in the post-anesthesia care unit (PACU). The patient’s nose, axilla and groin are swabbed as well as the area around the surgical site dressing. The bedrail is also swabbed. After the Case 2 patient is identified and consented, most of the same swabs are collected, excluding the baseline, surgeon and PACU swabs. Collecting swabs longitudinally to span the entire duration of the surgery allows us to determine if the pathogen transmission is occurring vertically, horizontally, or perioperatively. All swabs collected are sent to RDB Bioinformatics for ESKAPE pathogen analysis and data are reported to the central site.
After both surgeries, the patients’ deidentified information is input into the RDB database and submitted to the central site. This data entry involves an extensive review of the patients’ electronic medical record (EMR) to determine preexisting conditions (e.g., diabetes, hypertension, cancer, immunosuppression, etc.), case duration, ASA status, prophylactic antibiotic therapy and any possible complications that occurred from surgery (e.g., unplanned ICU admission).
90 days after surgery, the patient’s record is reviewed to identify any HAI or SSI that occurred. At our site, the hospital infection control team tracks and manages these data as part of national registries, so the data are easily available. For patients who developed HAI wound cultures or other laboratory testing would be reviewed to determine what kind of pathogen caused that infection and what medications were used to treat it.
Current Study Progress and Barriers to Enrollment
This study is primarily affected by two factors: the treatment courses prescribed by the surgeons and necessary staff to carry out the procedures outlined in the methods section. The wide geographical area captured in our patient population further presents a challenge to patients and surgeons when deciding whether to pursue surgical options. This variability can cause our caseload to fluctuate unpredictably depending on treatment outcomes for patients.
It is also challenging to enroll patients during such a busy time. Scheduling follow-up imaging, labs, and patient education take priority over study enrollment and make it difficult to connect with patients who are eligible for the study. This hectic workflow is illustrated in Figure 2 and highlights the perfect storm that must occur before patients can be consented to the study. To address these hurdles, we’ve established a team of undergraduate research volunteers who collectively spend 16 hours in the spine clinic, which ensures someone will be available to connect with patients who are recommended for surgical treatment. This team also collaborates to collect swabs in the operating room.
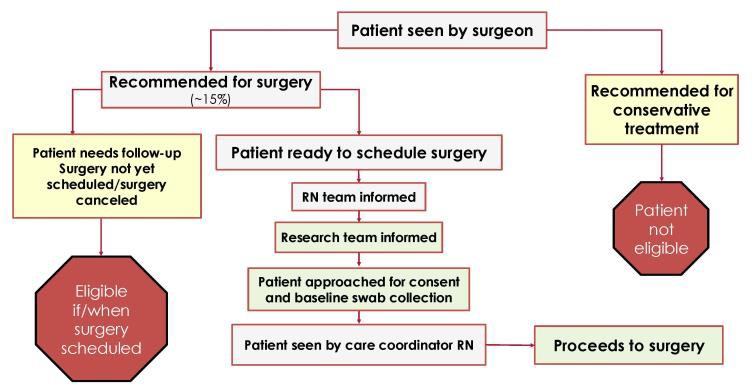
Figure 2. Spine Clinic Workflow
Despite these challenges, our site has enrolled over half of all patients enrolled at the 12 sites participating in the trial, demonstrating the challenges are not unique to our site.
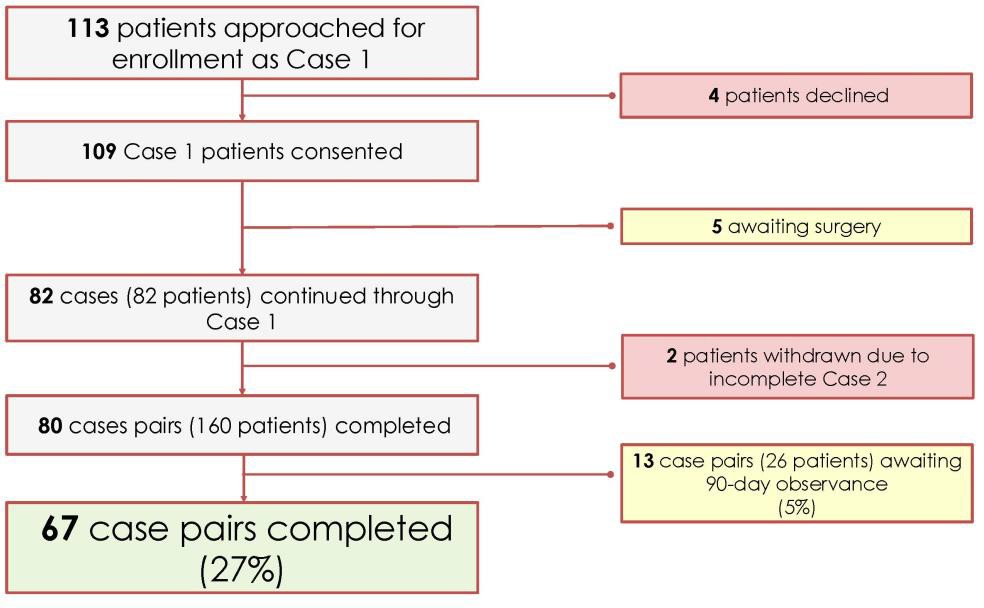
Figure 3: Current participant enrollment progress.
Results
Anticipated Data Analysis and Representation
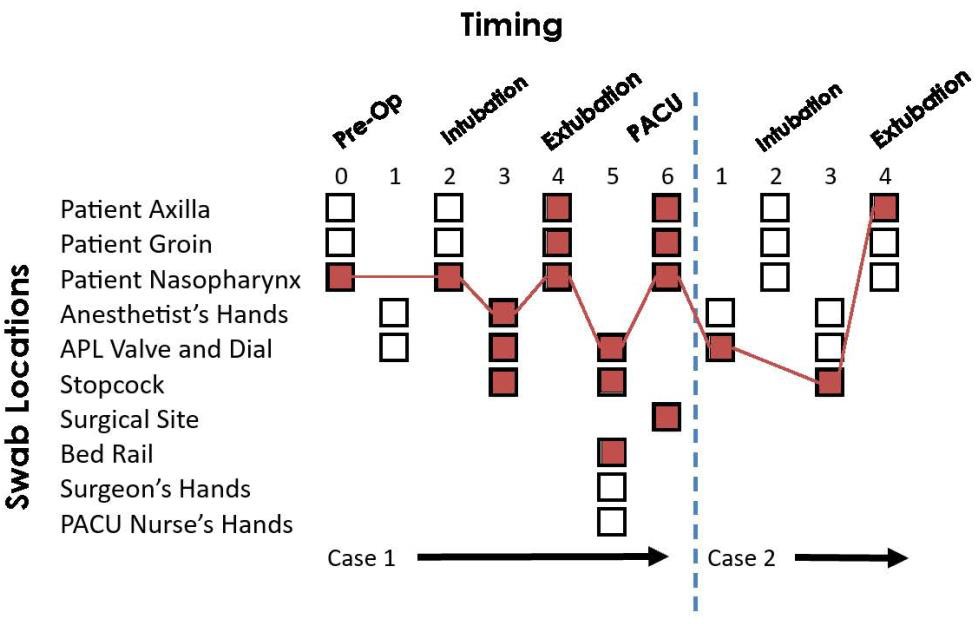
Figure 4. Hypothetical transmission map based on surveillance data collected from swabs.
Figure 4 is an example of how the transmission data collected from the swabs will be presented (as a control site, we do not have access to the surveillance/transmission data during data collection). Collecting swabs longitudinally throughout the duration of both surgical procedures allows a pathogen’s path to be traced through the operating room. In this figure, a pathogen is first collected in the Case 1 patient’s nose. This pathogen is then transmitted through various high-touch reservoirs and eventually ends up in the Case 2 patient’s axilla thereby putting them at risk for developing an SSI. A key observation from these data is that the transmission reservoir was the anesthetist’s workspace.
Figure 5 is a plot, taken from a previous study, the number of proportions of free wounds is decreasing at a faster rate without the infection-prevention bundle than with the bundle. This suggests that the infection-prevention bundle decreases the rate of SSI and increases the number of days a surgical site remains infection-free. In the context of the current study, data for the EBIP and/or surveillance with feedback will be graphed as the equivalent of the infection prevention bundle.
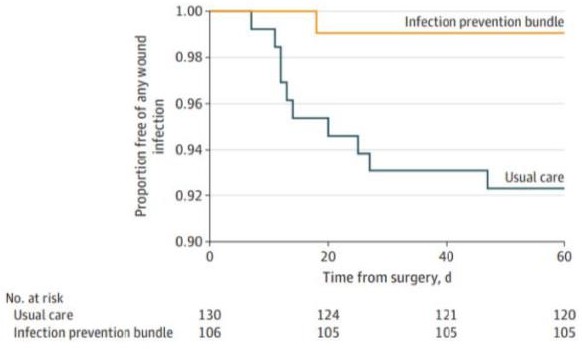
Figure 5: Kaplan-Meier plot used to visualize the proportion of infection free wounds over time.
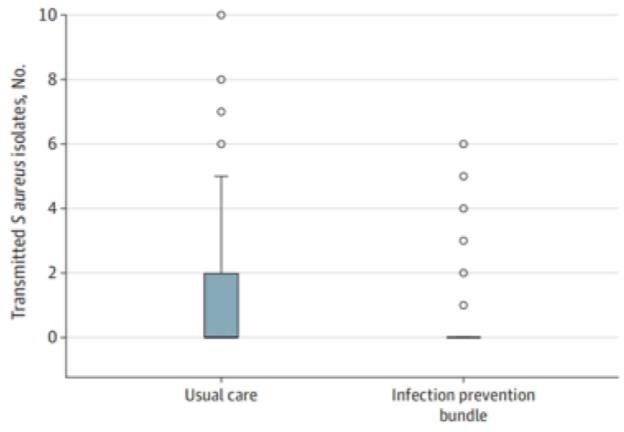
Figure 6: Transmission event barplot
Figure 6 demonstrates, from a previous study, the number of transmission events of S. aureus, one of the ESKAPE pathogens, decreased with the infection prevention bundle versus the usual care. In this study, we would expect to see a similar decrease in transmission events with EBIP with surveillance.Figure 7 organizes instances of SSI by surgical specialty and by intervention method. This is a hypothetical figure that aligns with the hypothesis of the study and demonstrates fewer instances of SSI with the EBIP with surveillance across both orthopedic joint and spine specialties.
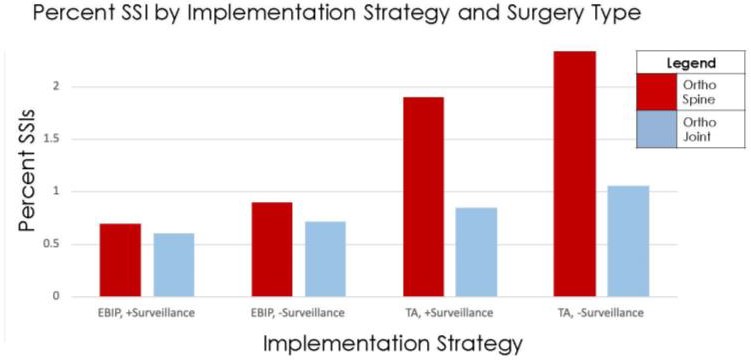
Figure 7: Hypothetical bar plot breaking down percent SSI by implementation strategy and surgery type
Discussion
We do not have access to transmission data at this time because the study is still ongoing. Additionally, because our site is assigned to the Technical Assistance (TA) without surveillance condition, we function as the control group. This design ensures that our team remains blinded to both intervention details and transmission data to preserve the integrity of the study’s findings.
Should the final data reveal that the combination of EBIP and Surveillance resulted in the lowest number of pathogen transmission events, it would substantially reshape our understanding of how to increase compliance with pathogen transmission within the operating room environment. This outcome would provide compelling evidence that a comprehensive approach, which includes team-based coaching and real-time surveillance, is essential for reducing transmission of ESKAPE pathogens. Such findings would emphasize the importance of high-touch areas, particularly in the anesthetist’s workspace and post-anesthesia care unit (PACU), as critical reservoirs for transmission.
Moreover, the implications for clinical practice would be significant. Beyond demonstrating the efficacy of EBIP and Surveillance, these results could inform the standardization of infection prevention protocols and guidelines in operating rooms. By
identifying specific high-risk areas and employing evidence-based implementation strategies to improve adherence to guidelines, this research could lead to better patient outcomes. Given the substantial financial burden associated with these infections, such advancements could dramatically improve also improve the efficiency of healthcare systems.
Finally, this study has broader implications for collaboration and education surrounding infection prevention protocols. The success of EBIP not only assesses the effectiveness of technical protocols but also fosters effective teamwork among healthcare providers, particularly anesthesiologists. This reinforces the importance of utilizing implementation strategies that prioritize education, engagement, and accountability within healthcare teams. By addressing both factors, this research could serve as a model for future studies aimed at reducing SSI and improving infection prevention practices across various clinical settings. Complication and side effect feedback to individual anesthesiologists is not a norm; if Infection Control Surveillance Data feedback to anesthesia departments or individuals is demonstrated to be effective in reducing cross transmission, then it could serve as a model for developing feedback systems to reduce other complications, such as acute kidney injury, cardiac complications, and postoperative readmission.
BIbliography
Project Details per NIH Reporter: https://reporter.nih.gov/search/K2pCU7vSnki4IlAz9Ghmxw/project-details/10618922 References
Brown, Jeremiah. “The BASIC Trial: Improving Implementation of Evidence-Based Approaches and Surveillance to Prevent Bacterial Transmission and Infection.” National Institutes of Health, U.S. Department of Health and Human Services, 17 June 2021, reporter.nih.gov/search/K2pCU7vSnki4IlAz9Ghmxw/project-details/10618922.
Dobson GP. Trauma of major surgery: A global problem that is not going away. Int J Surg. 2020 Sep;81:47-54. doi: 10.1016/j.ijsu.2020.07.017. Epub 2020 Jul 29. PMID:32738546; PMCID: PMC7388795.
Haque M, McKimm J, Sartelli M, Dhingra S, Labricciosa FM, Islam S, Jahan D, Nusrat T, Chowdhury TS, Coccolini F, Iskandar K, Catena F, Charan J. Strategies to Prevent Healthcare-Associated Infections: A Narrative Overview. Risk Manag Healthc Policy. 2020 Sep 28;13:1765-1780. doi: 10.2147/RMHP.S269315. PMID: 33061710; PMCID: PMC7532064.
Loftus, Randy W., Javier H. Campos,The anaesthetists’ role in perioperative infection control: what is the action plan?,British Journal of Anaesthesia, Volume 123, Issue 5,2019,Pages 531-534,ISSN 0007-0912, https://doi.org/10.1016/j.bja.2019.07.013.
Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079-1081. doi:10.1086/533452
Shepard J, Ward W, Milstone A, et al. Financial Impact of Surgical Site Infections on Hospitals: The Hospital Management Perspective. JAMA Surg. 2013;148(10):907– 914. doi:10.1001/jamasurg.2013.2246
Zimlichman E, Henderson D, Tamir O, et al. Health Care–Associated Infections: A Meta- analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern Med. 2013;173(22):2039–2046. doi:10.1001/jamainternmed.2013.9763

