John and Marcia Price College of Engineering
35 Effects of the Receptor for Advanced Glycation End Products on Successful Arteriovenous Fistula Maturation
River Tobias and Yan-Ting Shiu
Faculty Mentor: Yan-Ting Shiu (Bioengineering, University of Utah)
Introduction
Chronic kidney disease (CKD) affects approximately 37 million people in the United States, representing a significant health concern [1]. There are five stages to CKD, each determined by the decline of the glomerular filtration rate [2]. The fifth and final stage is called end-stage kidney disease (ESKD). This is when the kidneys have failed and can no longer filter blood effectively. ESKD is irreversible and expensive to treat, with 36 billion dollars being spent on ESKD in the US alone per year [1]. Of the 37 million Americans who have CKD, around 786,000 have ESKD and require renal replacement therapy [1]. Kidney transplants and hemodialysis are both common, but due to long wait times, 71% of ESKD patients use hemodialysis as their primary treatment [1].
Hemodialysis is a form of renal replacement therapy where blood is filtered outside the patient’s body using a dialyzer. To undergo this procedure, the patient needs a vascular access site that can handle the increased blood flow rate required by the dialyzer [3]. Among the various types of vascular access, the arteriovenous fistula (AVF) is preferred because it offers lower mortality and morbidity rates, as well as greater longevity compared to other options [4]. An AVF is created surgically by directly connecting the radial artery and cephalic vein of the wrist [4]. After AVF creation, the vein needs to undergo remodeling or build outwards to widen the lumen, allowing for the high blood flow needed to filter the blood properly. This remodeling process is called AVF maturation and should happen naturally after the AVF is created. However, while some AVFs can mature successfully for hemodialysis use, up to 60% of newly created AVFs in the US fail to mature [5]. One common form of AVF failure is due to neointimal hyperplasia formation, or the inward formation of tissue that narrows the lumen. This prevents proper access to the vascular site, and another site often is required [6]. This lengthy and challenging process can jeopardize patients’ access to life-saving treatment. There are currently few treatments to increase success in AVF maturation for ESKD patients. Treatments such as angioplasty or stents are available but are highly invasive [7]. Other treatments, such as nitric oxide (NO) and gene therapy, have some support as potential treatments, but more research on their effectiveness is still needed [8].
Inflammation and oxidative stress play important roles in vascular health. Advanced glycation end products (AGEs) and the receptor for advanced glycation end products (RAGE) increase oxidative stress due to increasing kinase and GTPase activity [9]. RAGE activity has previously been associated with decreased cardiovascular health and is a suspected factor in the progression of chronic kidney disease [10,11,12,13]. Additionally, these AGEs are not filtered out well by hemodialysis and are therefore found in greater concentration in patients with ESKD [14]. Due to their known roles in oxidative stress and increased fibrosis in the vasculature, it is possible that this could increase neointimal hyperplasia in the AVF, increasing the chances for maturation to fail.
Knowledge of a connection between RAGE and AVF health could provide a novel method to improve the success of essential treatment for ESKD patients. This study explores whether decreasing RAGE activity improves the AVF maturation process. Three mice subject groups were used in this experiment: untreated (or wild type), a gene knockout for RAGE, and a drug treatment to decrease RAGE activity. Surgically created AVF tissues were matured in each sample and were then collected to analyze both the formation of neointimal hyperplasia and fibrosis to measure the health of the AVF. This study gives insight into the effect of RAGE and AVF health during its maturation process through the inhibition of RAGE to determine whether further research into RAGE could lead to a possible treatment for hemodialysis patients. Ensuring that patients are able to receive hemodialysis treatment as soon as possible is imperative to their survival and quality of life.
Background
The kidney is an incredibly important organ. While it has many functions including producing vital hormones, one of its main functions is filtering out blood, removing harmful waste products such as urea, along with monitoring concentrations of ions and water. In chronic kidney disease, the function of the kidney in filtering blood is inhibited. Chronic kidney disease is a progressive disease characterized by 5 stages, with each stage characterized by the glomerular filtration rate, or the rate at which blood can enter the kidney glomerulus from the afferent arterioles [2]. The 5th and final stage, called end stage kidney disease or end stage renal disease, is when kidney function is reduced to the point that the patient could not survive, and the kidneys are considered to have failed [2]. This damage is irreversible. Factors that can lead to end stage kidney disease include diabetes and hypertension, and patients with cardiac issues are more likely to develop chronic kidney disease.
Current treatments for end stage kidney disease primarily include kidney transplant and hemodialysis. Kidney transplants have the lowest mortality rate and the best patient outcomes overall. However, many patients are not eligible for kidney transplants, and even for those who are, the average weight time for one is 49 months, which is too long to go without treatment [1]. For this reason, 71% of patients with end stage kidney disease have to undergo hemodialysis treatment [1].
Vascular access for hemodialysis
Hemodialysis is the process of filtering blood outside of the body, used to treat kidney failure. Patients usually undergo this therapy 3 times a week for up to 3-5 hours each visit [3]. Blood must first be collected from the body. The blood is filtered through thin hollow tubes inside a dialyzer [3]. The blood is then returned back to the body. A vascular access site needs to be created for blood both to be taken from and returned to the body, and it must be sufficient so as not to overwhelm the vasculature with the high blood pressure and flow leaving/returning to the body.
There are three main vascular access sites that are used. The first is a catheter, usually inserted directly into the jugular vein. The second method is an arteriovenous graft, a device that connects an artery to the vein, usually in the non-dominant arm. The third and final method is an arteriovenous fistula, or an AVF, which involves surgically creating a connection between a vein and an artery in the non-dominant arm [4]. Functional AVFs are less prone to infection and require less intervention than other vascular access types, reducing patient hospitalization and mortality [4]. When an AVF is created, several conditions need to be met before the AVF can become functional. First, the cross- sectional diameter needs to triple from 2 mm to 6 mm [15]. The flow also needs to increase considerably, going from 25 mL per minute to 500 [15]. This process is called AVF maturation or remodeling [5]. Unfortunately, 60% of newly created AVFs do not mature, especially in older patients [5]. When an AVF fails to mature, an intervention like angioplasty needs to be performed, or an entire new AVF needs to be created, both of which are intense burdens for patients and come with possible risks [7].
Advanced glycation end products
AGEs are stable posttranslational modifications of proteins formed by non- enzymatic reactions (through the Maillard reaction) with sugars such as glucose and related metabolites [9]. Unplanned spontaneous glycation of these metabolites changes their functions, often adversely, and can lead to cross-linking, aggregation, and the loss of enzymatic function, leading to decreased tissue function [9]. Not all AGEs cause these issues, but specific AGEs that have known complications are methylglyoxal (MG)-derived hydroimidazolone MG-H1, Nε-carboxymethyl-lysine (CML), and glucosepane [9]. It has been shown that AGEs are increased in patients with ESKD due to impaired renal clearance [14]. While AGEs can cause issues of themselves, their primary pathological effects result from binding to RAGE by enacting signaling cascades through binding to RAGE, which is a 45 kDa transmembrane receptor in the immunoglobulin family and expressed on the surface of many tissue cells, but most importantly including vascular wall cells [9]. The receptor characteristics allow it to bind to many different variations of AGEs, notably the known AGEs mentioned above [9].
When AGEs bind to RAGE, they can activate critical signaling pathways that increase oxidative stress, inflammation, and fibrosis [10,11,12,13]. One such pathway is the stimulation of nicotinamide adenine dinucleotide phosphate oxidase, thereby increasing the production of reactive oxygen species. RAGE is also involved in upregulating nuclear factor kappa B (NF-κB) [11]. NF-κB then leads to the transcription of specific genes such as endothelin-1, vascular cell adhesion molecule-1, intracellular adhesion molecule- 1, vascular endothelial growth factor, and E-selectin, increasing inflammatory responses in vascular tissue [11]. Taken together, the AGE-RAGE pathway leads to increased oxidative stress and inflammation in vascular tissues. Increased inflammation and oxidative stress are known contributors to neointimal hyperplasia, or the inward remodeling of vascular tissue [6]. This is an adverse reaction in a new AVF, as inward remodeling decreases blood flow and can make the AVF unusable. However, there is no direct link between RAGE activity and AVF maturation success, so a clear connection is still unknown.
Mouse model for RAGE expression
In vascular access research, several types of animal models have been used. For studies that look at physical properties such as shear stress or fluid dynamics, large animals are preferred, such as pig or sheep models [16]. However, for histological analysis, along with cell culture and protein analysis, small animal models such as mice and rats are preferred because the decreased cost enables more models to be used [16]. Furthermore, previous studies on treatments for AVF maturation have used mice models [16], so studies on mice models can be used as a more accurate comparison with previous studies for the efficacy of treatments.
Methods
To explore the effects of RAGE, mice models were used, and RAGE expression was decreased using two methods, both a gene knockout and a drug treatment, which were compared with a control group that had no treatment. After treatment to decrease RAGE, AVFs were surgically created in all mice groups and allowed to mature for a given time before the animals were sacrificed and the tissues were collected and processed for histology. Stained slides were created to analyze certain characteristics of the AVF vein to look for neointimal hyperplasia formation and overall vascular health to answer the question of RAGEs effect. Quantification was performed from the imaged slides, and statistical analysis was performed between each treatment group and the control group separately.
Animal Model and Group Allocation
A total of 30 mice were used for this study. Mice were divided into three groups: wild type untreated (control group), wild type treated with FPS-ZM1, a specific inhibitor of RAGE activity, and a full-body homozygous gene knockout (KO) for RAGE delivered intraperitoneally. C57BL/6 strain male mice around 3-4 months old were used for this study as the wild type.
Drug Treatment Protocol
Mice in the treated group were administered FPS-ZM1 at a dosage of 25 mg/kg body weight intraperitoneally. Twelve shots were administered to each mouse. The first injection was administered 5 days before AVF surgery, and was administered daily until 7 days after the AVFs were created.
Arteriovenous Fistula (AVF) Surgery
All mice underwent an arteriovenous fistula (AVF) surgery, where carotid-jugular AVFs were surgically created in young (3–4 month old) male mice to create the AVF, simulating those used in dialysis for end-stage kidney disease. Mice were anesthetized with isoflurane, buprenorphine, xylazine, and ketamine, and then a midline incision of the surgical area was performed. Using a surgical microscope, the right carotid artery and jugular vein were exposed. 10-0 monofilament microsurgical sutures were used to make a side-to-end anastomosis using the carotid artery and jugular vein. After unclamping, dilation of the vein and patency were confirmed visually. The mice were maintained on a warming blanket following surgery and buprenorphine was administered two times at 12 hours apart.
Tissue Collection and Histological Processing
After a maturation period of seven days, the mice were sacrificed, and AVF tissues were collected. The artery was then cut from the vein near the suture point, and the arteries and veins were processed separately to make embedding easier. Processing was done using a Leica TP1020 automated processor. Processing used the following reagents:
-Zinc-Buffered Formalin (Thermo Scientific, Waltham, Massachusetts, USA)
-70% Isopropanol (Fisherbrand, Waltham, Massachusetts, USA)
-95% Dehydration Alcohol (Fisherbrand, Waltham, Massachusetts, USA)
-100% Dehydration Alcohol (Harleco, Gibbstown, New Jersey)
-Xylene (Fisherbrand, Waltham, Massachusetts, USA)
-Liquid Paraffin Wax (Surgipath, Richmond, Illinois)
Tissues were then placed into cassettes to be embedded. The embedding machine used was Leica EG1150 H Tissue Embedding Center, with paraffin wax (Surgipath, Richmond, Illinois). The mold was chilled for at least 1 hour prior to sectioning with the Leica Rotary Microtome (model / part number). The mold boat was trimmed at 10 um thick slices until tissue could be observed. A different blade was then used to section the tissue and collect serial sections at 5 μm thick, placing them into a 38 °C water bath for 30 seconds to 1 minute before placing them on slides, where they were stored until staining. Each slide had 3 sections of tissue, and 2 slides were stained for each type of stain for each animal tissue sample. Typical samples had the first slide between 100 and 150 μm from anastomosis, and the second slide between 200 and 300 μm from anastomosis in most cases. Slides were imaged using the Zeiss Axioscan 7 (10X magnification).
Histological Staining and Imaging
In Masson’s Trichrome staining, sections were stained to evaluate fibrosis, with blue areas representing fibrotic tissue content, with specific protocol outlined in Appendix
A. In Verhoeff-van Gieson staining, elastic fiber staining was performed to evaluate neointimal hyperplasia, assessing the internal elastic lamina and open lumen area. The specific times and chemicals for the stain can be found in Appendix B. Slides for both stains were placed in a 55-60 degree Celsius oven for 12 hours before staining procedure.
Image Quantification
To quantify fibrosis on histological images, first photoshop (Version 21; Adobe Systems Incorporated) was used to mask the AVF out from surrounding tissue. Next, Image-J (Version 1.54k; Fiji) software was used to measure both the total area and the area stained blue in the AVF tissue. This was done using the color threshold, specifically the hue function while keeping all other factors constant in order to only select blue tissue. This hue value is different depending on the quality of the stain, but should be consistent for all sets of samples in a stain. Once an appropriate threshold was selected, the mean area was recorded. After this, hue was changed to include all values, which selects all of the tissue without selecting the background. Mean gray area was once again recorded. Equation 1 was then used to calculate % area fibrosis.
Equation 1: area % of fibrosis = mean gray area blue × mean gray area total
In the VVG stain, first a scale was set using the scale bar present in all imaged slides. In Image-J (Version 1.54k, Fiji), the length of the scale bar was recorded and set as the known distance. The internal elastic area shows up as a thin black line around the lumen in the VVG stain. The area was carefully traced in Image-J (Version 1.54k, Fiji), and this area was measured. Next, the actual open lumen was carefully traced. Equation 2 was used to calculate the area of the neointimal hyperplasia, equation 3 was used to calculate the normalized area of open lumen, and equation 4 was used to determine the percent area of open lumen. These equations were all implemented in Excel (v16.0, Microsoft).
Equation 2: 𝑎𝑟𝑒𝑎 𝑜𝑓 𝑙𝑒𝑠𝑖𝑜𝑛𝑠 = 𝑎𝑟𝑒𝑎 𝑒𝑛𝑐𝑙𝑜𝑠𝑒𝑑 𝑏𝑦 𝐼𝐸𝐿 − 𝑎𝑟𝑒𝑎 𝑜𝑓 𝑙𝑢𝑚𝑒𝑛
Equation 3: 𝑛𝑜𝑟𝑚𝑎𝑙𝑖𝑧𝑒𝑑 𝑎𝑟𝑒𝑎 𝑜𝑓 𝑜𝑝𝑒𝑛 𝑙𝑢𝑚𝑒𝑛 = area of open lumen × area of IEL
Equation 4: 𝑎𝑟e𝑎 % 𝑜𝑓 𝑜𝑝𝑒𝑛 𝑙𝑢𝑚𝑒𝑛 = 𝑛𝑜𝑟𝑚𝑎𝑙𝑖𝑧𝑒𝑑 𝑎𝑟𝑒𝑎 𝑜𝑓 𝑜𝑝𝑒𝑛 𝑙𝑢𝑚𝑒𝑛 [Equation 3] × 100%
Statistical Analysis
Data from treated groups (FPS-ZM1-treated and RAGE knockout) were compared to the wild type control group separately using a one-tailed Student’s t-test, since the only significant values would come from vascular health being improved in the treated groups. The study did not define a question relating knockout to drug treatment, so those groups were not compared to each other. The significance threshold was set at p < 0.05. Standard error (SE) and p-values were calculated from equation 5 to assess the significance of the differences in fibrosis and neointimal hyperplasia between groups. Excel (v16.0, Microsoft) was used in statistical analysis calculations.
Equation 5: SE =
σn−−√𝜎n
n = number of samples, σ = standard deviation
Ethical Considerations
All procedures involving animals were conducted in accordance with the guidelines of the University of Utah and were approved by the Institutional Animal Care and Use Committee (IACUC).
Results Section
To evaluate the impact of RAGE inhibition on AVF maturation, fibrosis levels and neointimal hyperplasia in three experimental groups: WT controls, FPS-ZM1-treated mice, and RAGE KO mice. From this, it was expected that RAGE KO would be the definitive group to demonstrate a complete absence of RAGE expression, and FPS-ZM1 would show limited RAGE expression in vascular tissues. Quantitative analysis was performed using histological staining and image processing techniques.
Neointimal hyperplasia was assessed from the imaged VVG stained slides. In Excel (v16.0, Microsoft), IEL area and lumen area were recorded from tracings performed in Image-J (Version 1.54k, Fiji), and from this the area of lesions, normalized area of open lumen, and area percent of open lumen, were calculated. Figure 1 highlights the results from the relationship between control AVFs and AVFs treated with FPS-ZM1. The average open lumen area percentage for the control group was 33.09%, which was higher than the drug treatment group at 24.31%. The p value was 0.229 which is far above 0.05, failing to indicate statistical significance. Standard error, calculated using Equation 5, demonstrated significant overlap between groups. Figure 2 highlights the results from the relationship between control AVFs and AVFs in KO mice. The average open lumen area percentage in the control AVFs was 33.09%, which was lower than the KO group at 36.47%. The p value was 0.455, which was above the 0.05 p value required to indicate statistical significance. Standard error similarly demonstrated significant overlap between control and KO groups.
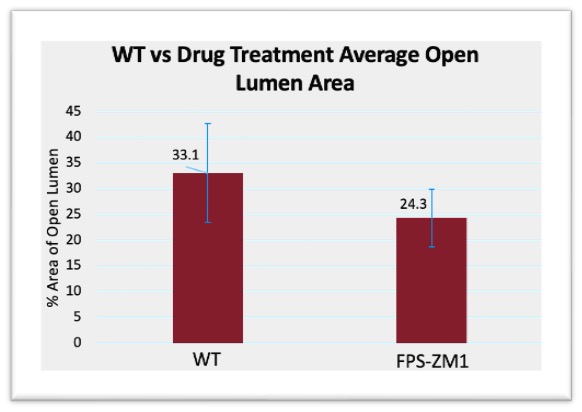
Figure 1: WT vs drug treatment open lumen area compared to total lumen area, p-Value =0.229. Error bars calculated using Equation 5, to demonstrate the range of data using standard deviation, and the error bars demonstrated significant overlap within a standard deviation between groups
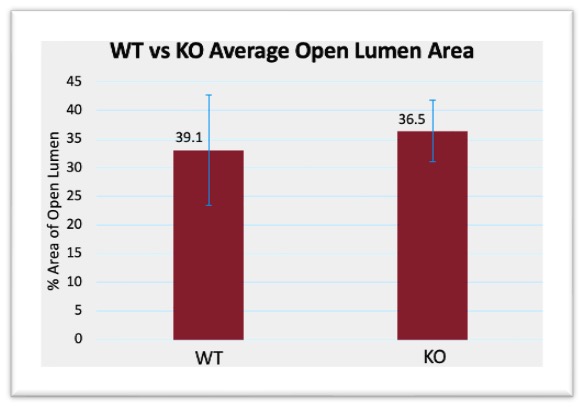
Figure 2: WT vs RAGE KO open lumen area compared to total lumen area, p-Value = 0.455. WT vs drug treatment open lumen area compared to total lumen area, p-Value = 0.229. Error bars calculated using Equation 5, to demonstrate the range of data using standard deviation, and the error bars demonstrated significant overlap within a standard deviation between groups
Masson’s Trichrome staining was used to evaluate fibrosis, with the percentage of fibrotic area measured relative to the total AVF area. As shown in Figure 3, average fibrosis in the FPS-ZM1-treated group was slightly increased compared to WT controls, but the p value was above 0.05 at p=0.206, and standard error demonstrated significant overlap between groups. Similarly, Figure 4 highlights that RAGE-KO mice demonstrated a slight increase in average fibrosis relative to WT controls, but the p-value was above 0.05 at p=0.163, and the standard error bars demonstrated significant overlap between groups.
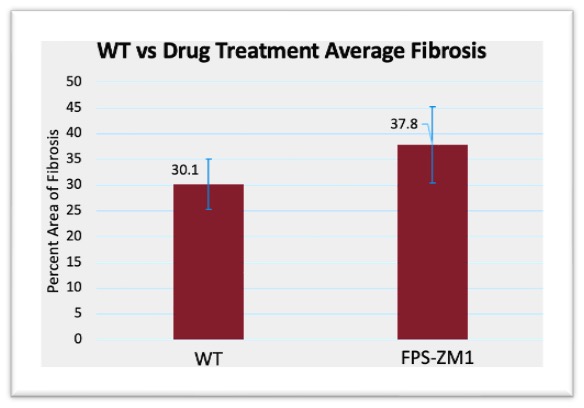
Figure 3: WT vs drug treatment fibrosis area compared to total AVF area, p-Value = 0.206. WT vs drug treatment open lumen area compared to total lumen area, p-Value = 0.229. Error bars calculated using Equation 5, to demonstrate the range of data using standard deviation, and the error bars demonstrated significant overlap within a standard deviation between groups
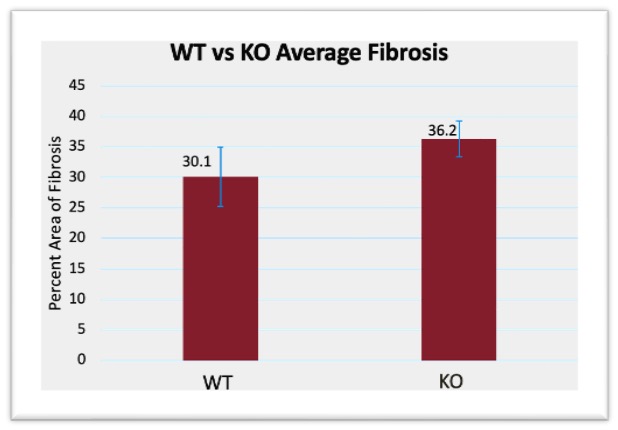
Figure 4: WT vs RAGE KO fibrosis area compared to total AVF area, p-Value = 0.163. WT vs drug treatment open lumen area compared to total lumen area, p-Value = 0.229. Error bars calculated using Equation 5, to demonstrate the range of data using standard deviation, and the error bars demonstrated significant overlap within a standard deviation between groups
Discussion
ESKD is an irreversible disease that requires immediate intervention, mainly through hemodialysis, for patients to survive. To ensure that patients can receive this care as soon as possible, it is imperative that the vascular access for hemodialysis works properly and is not the limiting factor. Unfortunately, 60% of AVFs, the most reliable form of vascular access, fail to mature properly. This paper aimed to determine whether decreasing the expression of RAGE in vascular tissue could improve AVF maturation success by improving vascular health. Mice animal models with AVFs were used, and a control group were compared to both a drug treatment (FPS-ZM1) to decrease RAGE, along with a RAGE knockout group. While some benefit was seen in the treatment groups, none of the results demonstrated statistical significance. Understanding the mechanisms behind vascular health is critical in improving AVF maturation success, thereby giving ESKD patients the best chance possible to live productive lives while managing their illness.
While many elements were quantified from the data, the most important indicators of AVF maturation success were the open lumen area percentage and the fibrosis percentage. Open lumen area percentage indicates how much of the lumen was actually usable for blood flow and was calculated using the IEL area compared to the observable open space. In healthy tissue, the IEL is the last barrier of the vascular wall, so the area encompassed by the IEL should be ideally made up entirely of ‘open space’ where blood can flow. By increasing the area of usable lumen in an AVF, there could be significantly more blood flow, allowing for hemodialysis to be effective. Percentage was considered more important than just overall lumen area, as the mice models had differences in vasculature, so overall vein size differed. Using percentage allowed for a normalized representation of the amount of usable lumen space in relation to the maximum potential. Figure 1 demonstrated a slight decrease in open lumen area in the drug treatment group compared to control, but Figure 2 demonstrated a slight increase in open lumen area for the KO group. Between the two groups, the KO was a more accurate representation of decreased RAGE expression. While FPS-ZM1 is a known RAGE antagonist, and its effectiveness has been demonstrated both in the brain and in vascular tissue, RAGE KO is more definitive, as it guarantees that no RAGE is present [17] [18]. Figure 2 highlights a possible relationship between decreasing RAGE and increasing open lumen area, albeit the differences were not statistically significant. However, the p value was over 0.05, so this result is not significant, as was the relationship between open lumen area in the drug treatment group.
While fibrosis content does not directly relate to the ability to draw a usable amount of blood from an AVF for hemodialysis, it nonetheless functions as an important indicator of overall AVF health. Increased fibrosis indicates an inability for a vein to recover from the stresses of maturation after AVF surgery. Veins with a greater degree of fibrosis are more prone to vascular disfunction and are often stiffer [19]. This can lead to improper remodeling, or even a late-stage failure where the vein cannot hold up and collapses after use. While some fibrosis is expected after an AVF surgery, minimizing fibrotic formation is desired to improve AVF success. In Figures 3 and 4, fibrosis was seen to increase. One possible reason is the treatments themselves. FPS-ZM1, while being a direct antagonist for RAGE, had significant side effects for the mice models used, and could have increased stress significantly. Similarly, a homozygous knockout was used for the KO group, which had significant side effects for the mice models. However, this increase in fibrosis compared to the control group was not significant for either the drug treatment group or the KO group, so a direct effect cannot be determined.
In previous studies, RAGE has been shown to be linked to increased oxidative stress and inflammation, notably through NF-κB and nicotinamide adenine dinucleotide phosphate oxidase, but other pathways and signaling molecules are involved in the negative effects seen from RAGE overexpression [11]. Additionally, previous studies have determined that AGEs are present in higher concentrations in ESKD patients, and while AGEs are not the only compatible ligand for RAGE, they are a significant factor in he overexpression of RAGE [14]. Many studies have also aimed to determine the relationship between RAGE expression and different aspects of health, and studies have found that RAGE is a significant factor in cancer progression, diabetes complications, and even arterial and vascular health [10], [11], [12], [13]. However, while many of these studies determined a connection between RAGE and upregulation of other factors known to affect vascular health, these studies did not try to decrease RAGE directly. Additionally, RAGE has connections to signaling molecules that are significant outside of AGEs that could be researched further. Sitruin-1 (SIRT1) is one that has had previous data support a connection to RAGE, both in previous studies and in current data in this research lab [20].
While previous studies have shown a relationship between RAGE and vascular health, the results from this study did not demonstrate an ability to control RAGE expression to improve vascular health. There are several possible limitations for this. One limitation is the methods used to decrease RAGE expression. Both drug treatment and KO had significant side effects outside of simply reducing RAGE in vasculature, which could have acted as confounding variables. Additionally, all the mice in this study were previously healthy, but a more accurate model would have animal models that had ESKD prior to AVF surgery, which would increase the AGEs in the bloodstream, possibly accentuating the effects of RAGE in the AVF. Finally, there were very few samples in many of the groups, specifically the control group, allowing possible outliers to confound results.
Moving forward, the animal samples that are being analyzed will be induced with CKD prior to surgery. Additionally, multiple types of KO treatment are being employed which should be more localized to vasculature. These changes, along with having more samples, could improve the current results and highlight a statistically significant relationship that could not be seen prior. Additionally, future directions outside of the project could aid in understanding how to improve vascular health in AVF maturation. Other related signaling molecules and pathways could be explored, such as SIRT1.
The ability to directly affect the outcome of AVF maturation success is critical to improving patients suffering from ESKD that need immediate treatment. To best help them, we must understand the full scope of what effects the maturation process, from flow rate to signaling pathways. Additionally, it is imperative to not only understand the relationship between variables and vascular health, but we must also be able to effectively control these variables for patients to have any benefit. Overall, this research is one step in gaining knowledge to help these patients and can hopefully be expanded on moving forward so that future treatment can improve patient outcomes for all those suffering from ESKD.
Reference
[1] “Kidney Disease Statistics for the United States – NIDDK,” National Institute of Diabetes and Digestive and Kidney Diseases. Accessed: Nov. 08, 2024. [Online]. Available: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease
[2] “Stages of kidney disease.” Accessed: Nov. 08, 2024. [Online]. Available: https://www.kidneyfund.org/all-about-kidneys/stages-kidney-disease
[3] “Hemodialysis – NIDDK,” National Institute of Diabetes and Digestive and Kidney Diseases. Accessed: Nov. 08, 2024. [Online]. Available: https://www.niddk.nih.gov/health- information/kidney-disease/kidney-failure/hemodialysis
[4] D. Santoro, F. Benedetto, P. Mondello, N. Pipitò, D. Barillà, F. Spinelli, C. Ricciardi, V. Cernaro, and M. Buemi, “Vascular access for hemodialysis: current perspectives,” IJNRD, vol. 7, pp. 281–294, Jul. 2014, doi: 10.2147/IJNRD.S46643.
[5] M. Allon, T. Greene, L. Dember, J. Vita, A. Cheung, N. Hamburg, P. Imrey, J. Kaufman, M. Robbin, Y. Shiu, C. Terry, H. Umphrey, and H. Feldman, “Association between Preoperative Vascular Function and Postoperative Arteriovenous Fistula Development,” Journal of the American Society of Nephrology, vol. 27, no. 12, p. 3788, Dec. 2016, doi: 10.1681/ASN.2015020141.
[6] A. Barac and J. A. Panza, “Mechanisms of Decreased Vascular Function With Aging,” Hypertension, vol. 53, no. 6, pp. 900–902, Jun. 2009, doi: 10.1161/HYPERTENSIONAHA.109.132308.
[7] J. H. M. Tordoir, N. Zonnebeld, M. M. van Loon, M. Gallieni, and M. Hollenbeck, “Surgical and Endovascular Intervention for Dialysis Access Maturation Failure During and After Arteriovenous Fistula Surgery: Review of the Evidence,” European Journal of Vascular and Endovascular Surgery, vol. 55, no. 2, pp. 240–248, Feb. 2018, doi: 10.1016/j.ejvs.2017.12.001.
[8] H. Hu, S. Patel, J. Hanisch, J. Santana, T. Hashimoto, H. Bai, T. Kudze, T. Foster, J. Guo, B. Yatsula, J. Tsui, A. Dardik, “Future research directions to improve fistula maturation and reduce access failure,” Seminars in Vascular Surgery, vol. 29, no. 4, pp. 153–171,
Dec. 2016, doi:10.1053/j.semvascsurg.2016.08.005.
[9] “Targeting the Receptor for Advanced Glycation Endproducts (RAGE): A Medicinal Chemistry Perspective | Journal of Medicinal Chemistry.” Accessed: Nov. 08, 2024. [Online]. Available: https://pubs.acs.org/doi/10.1021/acs.jmedchem.7b00058
[10] “Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression.” Accessed: Nov. 08, 2024. [Online]. Available: https://www.mdpi.com/1422- 0067/21/10/3613
[11] L. M. Senatus and A. M. Schmidt, “The AGE-RAGE Axis: Implications for Age- Associated Arterial Diseases,” Front. Genet., vol. 8, Dec. 2017, doi: 10.3389/fgene.2017.00187.
[12] J. Lee, J.-S. Yun, and S.-H. Ko, “Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus,” Nutrients, vol. 14, no. 15, p. 3086, Jul. 2022, doi: 10.3390/nu14153086.
[13] “Advanced Glycation End Products (AGEs) and Chronic Kidney Disease: Does the Modern Diet AGE the Kidney?” Accessed: Nov. 08, 2024. [Online]. Available: https://www.mdpi.com/2072-6643/14/13/2675
[14] N. Rabbani and P. J. Thornalley, “Advanced glycation end products in the pathogenesis of chronic kidney disease,” Kidney Int, vol. 93, no. 4, pp. 803–813, Apr. 2018, doi: 10.1016/j.kint.2017.11.034.
[15] M. Kokozidou, A. Katsargyris, E. L. G. Verhoeven, and G. Schulze-Tanzil, “Vascular access animal models used in research,” Annals of Anatomy – Anatomischer Anzeiger, vol. 225, pp. 65–75, Sep. 2019, doi: 10.1016/j.aanat.2019.06.002.
[16] M. Kokozidou, A. Katsargyris, E. L. G. Verhoeven, and G. Schulze-Tanzil, “Vascular access animal models used in research,” Annals of Anatomy – Anatomischer Anzeiger, vol. 225, pp. 65–75, Sep. 2019, doi: 10.1016/j.aanat.2019.06.002.
[17] F. Yang, Z. Wang, J. Zhang, J. Tang, X. Liu, L. Tan, Q. Huang, and H. Feng, “Receptor for Advanced Glycation End-Product Antagonist Reduces Blood–Brain Barrier Damage After Intracerebral Hemorrhage,” Stroke, vol. 46, no. 5, pp. 1328–1336, May 2015, doi: 10.1161/STROKEAHA.114.008336.
[18] R. Deane, I. Singh, A. Sagare, R. Bell, N. Ross, B. LaRue, R. Love, S. Perry, N. Paquette, R. Deane, M. Thiyagarajan, T. Zarcone, G. Fritz, A. Friedman, B. Miller, and B. Zlokovic, “A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in
a mouse model of Alzheimer disease,” J Clin Invest, vol. 122, no. 4, pp. 1377–1392, Apr. 2012, doi: 10.1172/JCI58642.
[19] A. Harvey, A. C. Montezano, R. A. Lopes, F. Rios, and R. M. Touyz, “Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications,” Can J Cardiol, vol. 32, no. 5, pp. 659–668, May 2016, doi: 10.1016/j.cjca.2016.02.070.
[20] K.-P. Huang, C. Chen, J. Hao, J.-Y. Huang, P.-Q. Liu, and H.-Q. Huang, “AGEs-RAGE System Down-Regulates Sirt1 Through the Ubiquitin-Proteasome Pathway to Promote FN and TGF-β1 Expression in Male Rat Glomerular Mesangial Cells,” Endocrinology, vol. 156, no. 1, pp. 268–279, Jan. 2015, doi: 10.1210/en.2014-1381.
Appendix A: Masson Trichrome Protocol
Deparaffinization and rehydration
-Xylene for 5-6 min (Fisherbrand, Waltham, Massachusetts, USA) Repeated with two more additional containers of Xylene for 5-6 min
-100% EtOH for 5 min, then repeated with a second container (Harleco, Gibbstown, New Jersey)
-95% EtOH for 5 min (Fisherbrand, Waltham, Massachusetts, USA)
-80% EtOH for 5 min (Fisherbrand, Waltham, Massachusetts, USA)
-Two changes of diH2O
In the first change, held the slides in the water for 3 min. If staining 24 slides, held in for 4 min.
In the second change, held the slides in the water for 4 min. If staining 24 slides, held in for 5 min.
Bouin’s Solution
-Submerged the slides in Bouin’s solution and placed the container in the water bath (60 °C) for 2 hr (Sigma-Aldrich, St. Louis, Missouri)
-After 2 hr, tissues were transferred to a container with running tap water for approximately 1 min
-2 containers of diH2O for 30 sec Dye Application
-Biebrich-Scarlet Acid Fuchsin for 8-8.5 min (Chromaview)
-5 changes of diH2O. Five dips in each change of diH2O
-Phosphotungstic-phosphomolybtic acid for 5 min (Sigma-Aldrich, St. Louis, Missouri)
-Aniline Blue for 5-5.5 min (Sigma-Aldrich, St. Louis, Missouri)
-1% Acetic Acid for 2-2.5 min (Fisherbrand, Waltham, Massachusetts, USA)
-3 changes of diH2O three times for about Dehydration, Clearing, and Mounting
-5 dips in 95% EtOH (Fisherbrand, Waltham, Massachusetts, USA)
-5 dips in 100% EtOH for 2 changes (Harleco, Gibbstown, New Jersey)
-Xylene for at least 3 min (Fisherbrand, Waltham, Massachusetts, USA)
-Mounted the slides using Toluene Permount Solution and coverslip (Fisherbrand, Waltham, Massachusetts, USA)
Appendix B: Verhoff Van Gieson Protocol
Deparaffinization and rehydration
-Xylene for 5-6 min (Fisherbrand, Waltham, Massachusetts, USA) Repeated with two more additional containers of Xylene for 5-6 min
-100% EtOH for 5 min, then repeated with a second container (Harleco, Gibbstown, New Jersey)
-95% EtOH for 5 min (Fisherbrand, Waltham, Massachusetts, USA)
-80% EtOH for 5 min (Fisherbrand, Waltham, Massachusetts, USA)
-Two changes of diH2O
In the first change, held the slides in the water for 3 min. If staining 24 slides, held in for 4 min.
In the second change, held the slides in the water for 4 min. If staining 24 slides, held in for 5 min.
Dye Application
-Elastic Stain solution for 15 min (ThermoView, Louisville, Kentucky)
-5 separate changes of diH2O 1st change: 5 dips
2nd change: 5 dips 3rd change: 8 dips 4th change: 8 dips 5th change: 8 dips
-Ferric Chloride solution for 2.5 min (ThermoView, Louisville, Kentucky)
-Running lukewarm tap water for 30 sec
-95% EtOH for 2 min (Fisherbrand, Waltham, Massachusetts, USA)
-diH2O for 30 sec
-Van-Gieson solution for 3 min (Thermoview, Louisville, Kentucky)
Dehydration, Clearing, and Mounting
-5 dips in 95% EtOH (Fisherbrand, Waltham, Massachusetts, USA)
-5 dips in 100% EtOH for 2 changes (Harleco, Gibbstown, New Jersey)
-Xylene for at least 3 min (Fisherbrand, Waltham, Massachusetts, USA)
-Mounted the slides using Toluene Permount Solution and coverslip (Fisherbrand, Waltham, Massachusetts, USA)
Media Attributions
- 137489262_figure1
- 146879582_figure2
- 146879585_figure3
- 146881378_figure4

