College of Social and Behavioral Science
114 Paleoecological Coprophilous Spore Analysis Reveals Disturbance of Pastoralists in the Fynbos Biome, Cape Floristic Region, South Africa.
Bennett Davenport; Stella Mosher; and Mitchell Power
Faculty Mentor: Stella Mosher (Anthropology, University of Utah)
Background
The Cape Floristic Region (CFR) of South Africa, home of the Fynbos Biome, provides one of the most biodiverse ecosystems on the planet, which has for millennia been impacted by human habitation. Critically, pastoralists migrated into the region and introduced massive populations of herbivorous livestock around 2000 years ago (Skead, 2011). The most significant archeological evidence from this arrival comes from sheep bones in Tortoise Cave, near Eland’s Bay, which have been dated to 1530±122 cal years BP (Klein, R., & Cruz-Uribe, K., 1987). The magnitude of these herds and more evidence of the time of arrival are left obscured – a paleoecological proxy that could assist in answering these questions is that of coprophilous spores. The reliance on the digestive tracts of herbivorous animals means the spores of coprophilous fungi can be indicators of herbivore populations (Lee et al., 2022). Additionally, the spores do not travel far, usually landing a couple of meters away from the fruiting body of the fungus (van Asperen et al., 2021). As well, they are most effective at tracking large herbivores (such as livestock) due to the fact that large herbivores will produce a greater quantity of dung (Lee et al., 2022). For these reasons, coprophilous spores can be excellent indicators for local populations of large herbivores. In the past, coprophilous spores have been used as a proxy for megafaunal herbivore populations in North America during the late Quaternary (van Asperen et al., 2021). Coprophilous spores have also been used in European paleoecology research (Goethals et al., 2019), with only a few select studies using the proxy for any region of the African Continent (Goethals et al., 2019; Burney et al., 2003; Ekblom et al., 2010).
The knowledge of these African spore assemblages remains very limited (Ekblom et al., 2010). One of the most common spores quantified in the palaeoecological literature is Sporormiella because of its identifiable appearance and application as an indicator of herbivore presence (Lee et al., 2022). Although reliable, other spore taxa, such as Coniochaeta, Sordaria, etc, are heavily recommended to be measured along with Sporomiella, especially in studies exploring ecosystems where very little spore research has been done (Ekblom et al., 2010; Lee et al., 2022). Thus, an analysis of coprophilous spores as a proxy in the CFR should also include an analysis of viable spore taxa of the region that can be used. My research set out to analyze the pastoralist impact on the Fynbos Biome using coprophilous spore analysis, coming from dated sediment samples from the Fynbos Biome: Eilanvlei and Verloenvlei, located on the southern Cape coast and west coast, respectively. The final result would yield important paleoecological data about the introduction of pastoralists and their livestock into the Fynbos region, and the magnitude of such migration through the lens of the coprophilous spore record, both important variables in understanding human impact on this biodiverse region.
MATERIALS & PROCEDURE
Coprophilous spore samples came from the sediment cores taken from Verlorenvlei and Eilandvlei of the CFR. Sediment samples used for the counting of the CFR’s coprophilous spore assemblage came from the historic Eilvandvlei Core, while the samples used for the analysis of herbivore populations came from the older, “long” core from Verlorenvlei. The samples from this core extended further back in time, approximately 1900-1500 CAL BP. The samples were prepared in the Paleobiology Lab at the Natural History Museum of Utah. Sediment samples are collected and added to test tubes, along with a tablet of Lycopodium spores that are referenced during counting. The samples are then reacted with hydrochloric acid, centrifuged, and decanted multiple times before being soaked in a hot water bath. They are then sieved using a 125-micron sieve and transferred to another set of test tubes. Then the samples are subjected to several more cycles of chemical reaction, stirring, centrifugation, and decantation before finally being stained and stored in a desiccator. Under the microscope, the Lycopodium spores are counted alongside the sample’s coprophilous spores. This is used to analyze the relative abundance of coprophilous spores to the Lycopodium spores, which is the easiest way to analyze and compare spore abundances in these samples.
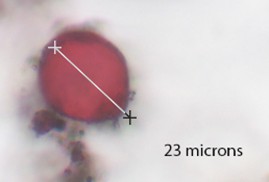
Three slides from the Eilandvlei core were produced and analyzed, with spores photographed and later identified. Of these, seven coprophilous spore taxa were identified in these Eilandvlei samples, which were used in the historic Verlorenvlei samples, depicted below. Later, 10 slides were prepared from the Verlorenvlei core. The spores identified above were counted alongside Lycopodium spores, with the relative abundance of each spore, and combined coprophilous spores tallied.
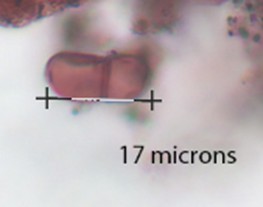
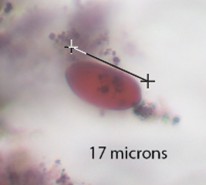
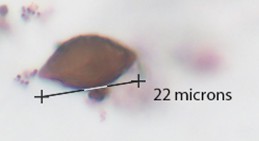
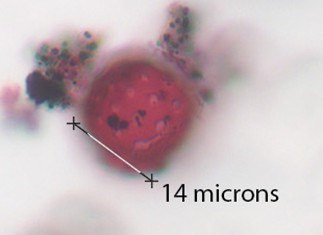
Table 1: Diagnostic coprophilous spores identified in the study
|
Sporormiella |
Dark in color, made of 4 cells, usually split apart under acetolysis. Every cell can contain a sigmoid aperture. |
|
|
Sordaria |
Small oblong spore, may look deformed or wrinkled due to aceytolysis. Dark in coloration. |
|
|
Podospora |
Pumkin seed-shaped spore, thick brown membrane. About 20-30 microns in size. |
|
|
Coniochaeta |
Small round cell around 10 microns in diameter, visible and sometimes frayed or deformed outer layering. |
|
|
Gelasinospora |
Round cell about 25 microns in diameter, ridged cell membrane, pores throughout the surface. |
|
|
Chaetomium |
Round, pink spore, with a small protrusion on one side like an orange. Can be described as “lemon shaped”. ~10 microns in length. |
*example seems considerably oversized |
|
Delitschia |
Ascospores ellipsoid, 2-celled, roughly 30 × 15 microns in size, thick-walled, and pinched around the middle. |
NOT PICTURED |
RESULTS
The ten samples from the Verlornvlei long core are presented below. Relative abundance was calculated as the number of spores over the number of Lycopodium.
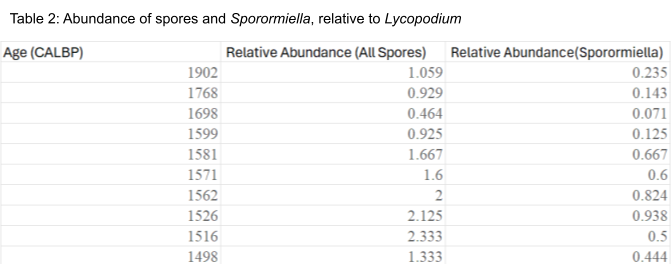
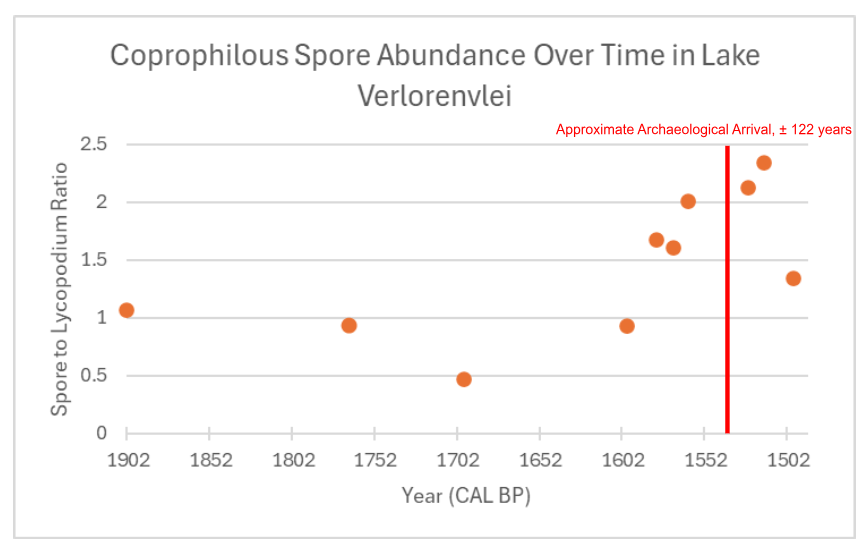
Figure 1: Archaeological arrival taken from (Klein, R., & Cruz-Uribe, K., 1987)
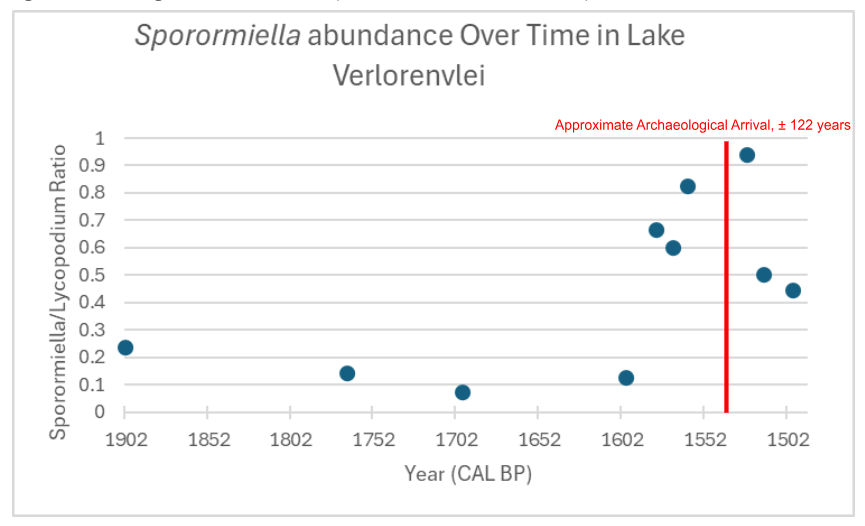
Figure 2: Archaeological arrival taken from (Klein, R., & Cruz-Uribe, K., 1987)
Spore relative abundance plateau from years 1902 to 1602 cal year BP before increasing and peaking at 1516 cal year BP, increasing to an abundance 100% larger than the original stagnant period abundances (Table 2). After 1516 cal year BP, abundances show a dropoff, although they still remain higher than before the year 1602. Welch’s two-sample T-test was performed on the data, where the samples were separated into “before” and “after” categories depending on their position in a specified year. A T-test using the year 1530 cal year BP gave a P-value of 0.0658, therefore not statistically significant. However, using the years 1570 up to 1600 cal year BP, all yield p-values below 0.05, thus disproving the null hypothesis.
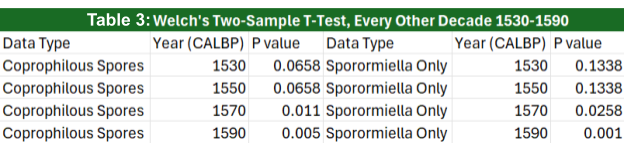
What is still notable is that the two highest numbers of relative abundance occur ~5 and~15 years after 1530 cal year BP. It should also be noted that Welch’s two-sample T-test has similar results between relative spore abundances using all spore taxa as well as the relative abundance of just Sporormiella. Sporormiella relative abundance peaks ~5 years after 1530 cal year BP.
CONCLUSION
The data from coprophilous spore relative abundance analysis appears to support a significant ecological change within the populations of large herbivores, which aligns with archeological evidence of pastoralists entering Cape Province. Specifically, the abundances match a pastoralist introduction between 1600 and 1500 cal year BP. The findings do not detect the introduction of pastoralists during 1530 cal year BP; the mean age of the livestock remains from previous literature. Instead, data instead detects the emergence of livestock herds that precede 1530 cal year BP by at least 30 years. Chronologic errors with coprophilous spore data have been noted before and may be due to diagenetic processes experienced by the sediment samples. The relative abundance “peaking” around 1530 must also be questioned, as spore counting is inaccurate as a direct proxy for herbivore population size (van Asperen et al., 2021). Yet it is still noteworthy that the archeological evidence correlates not with the initial increase in abundance, but with the largest relative abundance the samples yielded. Overall, the data seems to reveal a pastoralist introduction several decades before the assumed archeological date.
The correlation between the combined spore assemblage abundances and the Sporomiella-only abundances is a vital scientific takeaway. As Sporormiella remains one of the most established and trusted coprophilous spores for spore counting, the fact that data collected from the other spore taxon of the Fynbos match the patterns of Sporormiella supports an argument that these other spore taxons are of equal importance. Hopefully, the data collected in this paper will help deepen the understanding of the coprophilous spore taxa of South Africa, as well as prove the potential of analyzing the coprophilous spore taxa outside of Sporormiella. Overall, the data collected should reveal the importance of the field of spore counting when applied to paleoecological contexts in which it is absent. It is advised that these analyses be replicated, at a more intense gradient, in order to produce a more accurate and solidified interpretation of the paleoecological timeline.
Bibliography
Burney, D. A., Robinson, G. S., & Burney, L. P. (2003). Sporormiella and the late Holocene extinctions in Madagascar. PNAS, 100(19), 10800-10805.
Ekblom, A., & Gillson, L. (2010). Dung fungi as indicators of past herbivore abundance, Kruger and Limpopo National Park. Palaeogeography, Palaeoclimatology, Palaeoecology, 296(1-2), 14-27. https://doi.org/10.1016/j.palaeo.2010.06.009
Goethals, L., & Verschuren, D. (2019). Tracing ancient animal husbandry in tropical Africa using the fossil spore assemblages of coprophilous fungi: a validation study in western Uganda. Vegetation History and Archaeobotany, 29(5), 509-526. https://doi.org/10.1007/s00334-019-00760-3
Klein, R., & Cruz-Uribe, K. (1987) Large mammal and tortoise bones from elands bay cave (South Africa) Bar International Series, 322, 350-372
Lee, C. M., van Geel, B., & Gosling, W. D. (2022). On the Use of Spores of Coprophilous Fungi Preserved in Sediments to Indicate Past Herbivore Presence. Quaternary, 5(3). https://doi.org/10.3390/quat5030030
Skead, C. J. (2011). Man’s influence on the incidence of the larger mammals. In Historical incidence of the larger land mammals in the broader Western and Northern Cape. Centre for African Conservation Ecology, Nelson Mandela University.
van Asperen, E. N., Perrotti, A., & Baker, A. (2021). Coprophilous fungal spores: non-pollen palynomorphs for the study of past megaherbivores. Geological Society
Media Attributions
- Davenport fig1_image
- davenport_fig2_image