Spencer Fox Eccles School of Medicine
71 Evaluating a Social Anxiety Neural Pathway: Silencing BNST To NAc Projecting Neurons Before Behavioral Assay
Caitlin Tweed and Moriel Zelikowsky
Faculty Mentor: Moriel Zelikowsky (Neurobiology & Anatomy, University of Utah)
ABSTRACT
The nucleus accumbens (NAc) is traditionally known for its involvement in motivation. In recent literature, there has been a shift in focus to specifically social motivation (Trezza et al., 2011). The NAc contains neurons which lead to two opposing downstream paths – controlling both appetitive social behaviors and social avoidance behaviors(Kai et al., 2015). The Bed Nucleus of the Stria Terminalis (BNST) region of the brain, known for its role in anxiety behaviors, projects to a small portion of neurons in the NAc, therefore the activity of the BNST can affect the activity of the NAc (van de Poll et al., 2023). This study explores the necessity of BNST projections to the NAc in social anxiety behaviors displayed by mice who have experienced long-term social isolation.
Using a chemogenetic approach BNST to NAc projections were silenced in both isolated and group housed mice, before social behavioral experimentation. We found that certain social behaviors, such as social fear and social motivation, increased, while social hesitancy behaviors were reduced. This work further characterizes and identifies the connections between the BNST and the NAc. BNST to NAc projections play an important role in isolation induced social anxiety behaviors, but further research is necessary to determine how subpopulations of neurons control the individual behaviors associated with social anxiety. Ultimately, this work expands what is known about isolation induced social anxiety, a constant struggle faced by many that has become increasingly prevalent in the past decade, and the neural activity associated with anxiety- linked social behaviors (Loneliness in America, 2025).
Introduction
Social anxiety affects 12% of Americans over their lifetime, and the effects of mass social isolation on individuals following the COVID-19 global pandemic are recently coming to light (National Institute of Mental Health (NIMH), n.d.). This study expands what is known about isolation induced social anxiety and the neural activity associated with social behaviors resulting from this isolation. It provides insight into how individuals who were socially and mentally affected by the pandemic, or other isolating experiences, may have been affected neurally. A 2021 Harvard report found 36% of Americans were experiencing extreme loneliness (LonelinessinAmerica, 2025). Whether that loneliness is due to their physical location, a loss in their life, or falling into a plethora of other hardships and coping through isolation, loneliness can lead to the development of social anxiety. In addition to the mental toll of the pandemic and other isolating situations, there has been a rise in diagnoses of social aversion disorders such as social anxiety, PTSD, and autism spectrum disorder (Goodwin et al., 2020; Sareen, 2014). Social connections are not only critical to the mental and emotional health of social species, such as mice or humans, but they also have a positive impact on physical health (Holt-Lunstad, 2024). The neural pathways underlying social anxiety behaviors, especially those consequent of social isolation, have yet to be fully investigated. Understanding these pathways is essential to developing treatments and therapies for these widespread disorders that affect many.
The Nucleus Accumbens (NAc)
Within the past 20 years, research on the nucleus accumbens (NAc) region of the brain has gained speed. Though a lot is yet to be discovered, it has been found and continually supported that the NAc receives reward signals coding for social motivation (Richey et al., 2014). The NAc is involved in social approach behaviors and dysfunctions of the neural pathways that include the nucleus accumbens play a role in social anxiety behaviors (Ma et al., 2020). These pathways, namely the mesocortical and mesolimbic, are particularly impactful on social motivation and anxiety when compared to the other major dopamine pathways of the central nervous system (Mathew et al., 2001). Activity decreases in neurons that synapse with the nucleus accumbens in cases of social apprehension and avoidance. A 2020 study on characteristics of neurons in the nucleus accumbens after experiencing “social defeat” found the physical structure of the neural dendrites had altered (Fox et al., 2020).
The Bed Nucleus Stria Terminalis (BNST)
While research on the NAc has been blossoming, so has the investigation of the bed nucleus of the stria terminalis (BNST) region of the brain. The BNST contains neurons with receptors to stress hormones and then sends outputs to brain structures that ultimately produce sustained fear responses (Davis et al., 2010). As it is associated with sustained fear, rather than startle responses and momentary stress, the BNST plays an important role in anxiety, “a sustained state of apprehension”, pathways (Walker et al., 2003). fMRI imaging of the human brain revealed that neurons in the BNST region were excited when participants experienced anxiety or were expecting anxiety-causing stimuli (Lebow & Chen, 2016).
Connecting the BNST to the NAc
Many neurons originating in the BNST project to those in the nucleus accumbens, creating neural pathways that extend through both regions (Xiao et al., 2021). The connections between a region known for its role in anxiety, the BNST, and a region known for its role in social motivation, the NAc, have yet to be thoroughly investigated. In this study, the activity of the targeted BNST to NAc projecting neurons was manipulated and the changes in social behaviors were observed and analyzed. In order to also observe the role of these neurons after developing in social isolation, both socially isolated and group housed mice underwent the same neural silencing and behavioral assay. The general objective of this study was to investigate the role of BNST to NAc projecting neurons. Specifically, we evaluated the effect of silencing these neurons on the social anxiety phenotype in both socially isolated and group housed c57 mice. Silencing the connections between the BNST region and the NAc and then studying social behavior in isolated mice provides a foundation of neural knowledge concerning social anxiety pathways and associated behaviors to be built upon.
Our work on researching the BNST’s role in social isolation induced anxiety has supported us in this next goal of further characterizing and identifying the connections between the BNST region and the NAc. It is unknown how necessary functionally and anatomically BNST projections to the NAc are for social anxiety behaviors to be displayed in mice who have experienced long-term isolation. Through dual virus injections that target BNST to NAc projections using a chemogenetic approach BNST neurons were silenced. Original behavioral experimentation, called Selective Access to Unrestricted Social Interaction (SAUSI), then revealed an isolated mouse’s presentation (or lack) of the social anxiety phenotype (Grammer et al., 2024).
Methods
Phase 1: Isolation
The C57BL/6 mouse was the selected model due to their genetic and inherited phenotypic uniformity (C57BL/6Mice, n.d.). They are also, like other mice strains, social creatures that show interest in interacting with others (Netser et al., 2020). All mice (n=24) were raised in a group housed setting before being randomly selected to be either group housed or isolated at six weeks old. Half of the sample were removed from their home cage and socially isolated (SI) (n=12) and the other half remained group housed (GH) (n=12).
Phase 2: Intracranial Injections
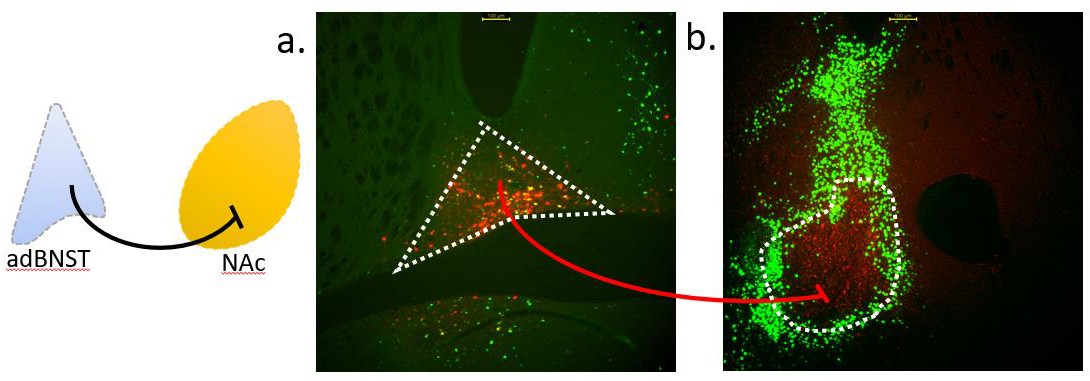
Three to seven days following isolation, each mouse received 2 sets of bilateral intracranial injections. After denoting the bregma point on the skull as the zero position, two craniotomies were performed in order to access the nucleus accumbens (NAc) at the anterior/posterior coordinate +1.98; one at the medial/lateral coordinate +0.75 and the other across from it at the medial/lateral coordinate -0.75. Two more craniotomies were performed to provide access to the BNST region. These were situated more posteriorly on the skull at an anterior/posterior coordinate of +0.95 and had medial/lateral coordinates of +/- 0.85 (Figure 2). Once all 4 craniotomies were made, 200 nL of a cre containing, retrograde virus (retropENN.AAV.hSyn.HI.eGFP_ cre.WPRE.SV40) was injected at a rate of 50 nL/min through each anterior craniotomy positioned into the NAc bilaterally (at an A/P position of +1.98). It was placed at a dorsal/ventral position of -4.5. This virus is cre dependent, and retrograde traveling. At each posterior craniotomy position (at an A/P position of +0.95), 150 nL of a cre-dependent inhibitory virus coding for Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) (AAV2-hSyn-DIO- hm4di-mcherry) was injected at 50 nL/min into the BNST bilaterally. It was placed at a dorsal/ventral position of -4.1. Before surgery mice were administered carprofen at a dose of 5 mg/kg. Directly following surgery mice were provided additional analgesia to aid in healing (buprenorphine XR at a dose of 2.5mL/kg).
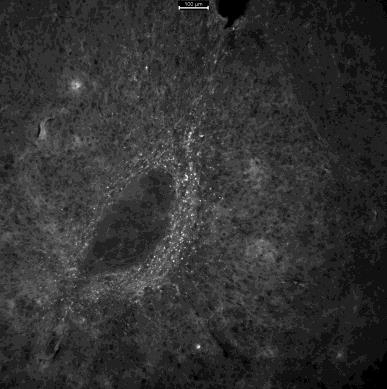
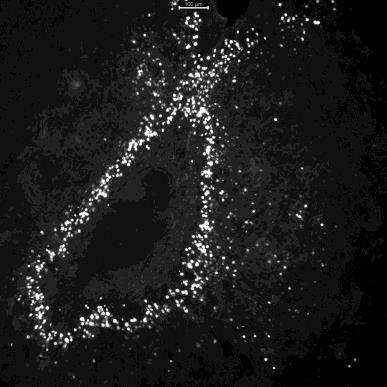
In order to target BNST to NAc projecting neurons, cre was used. The virus injected into the NAc is expected to travel backwards through the neurons with terminals in the NAc. As the BNST-injected virus requires cre to express, it will only express in neurons that terminate in the NAc, where a cre containing, retrograde virus was injected. An experiment to test these viruses paired together was conducted in advance, and microscopy images proved successful viral expression (Figure 1). Neurons in the BNST that project beyond the NAc primarily expressed GFP. Viral expression of neurons projecting from the BNST to the NAc was tagged with mCherry.
Phase 3: Social Behavioral Experimentation
Four weeks were required for complete viral expression and social isolation. At 10 weeks old, all mice underwent an original behavioral assay titled Selective Access to Unrestricted Social Interaction (SAUSI). SAUSI requires five days of individually placing the test mice in an enclosure designed for free movement between two chambers (Figure 2). On day one, mice were placed into the empty chamber for five minutes each. Every 30 seconds, the mouse was guided through the tube and every 15 seconds the experimenter’s hand waved through the opposite chamber. This desensitized the mice to the experimenter and prevents the association of the experimenter’s hand with being directly interacted with. On day two, the same process was repeated as mice adjusted to the enclosure. On day three, each test mouse was placed into the enclosure undisturbed for five minutes and the control BALB/c mice were trained to avoid entering the tunnel via the foot shock board.
Days four-five are the behavioral assay phase (Figure 3). On day four, test mice were injected with either Deschloroclozapine (DCZ) at a dose of 0.25 mg/kg, the ligand required to silence the targeted BNST to NAc neurons, or a control vehicle (DMSO and saline). Ten minutes after the injection, the mouse was placed into the enclosure for a three minute baseline phase. They were then kept in one chamber with the tube blocked off, as a control BALB/c mouse is placed in the opposite chamber. The tube is then unblocked and the test mouse is given unrestricted access to both chambers for ten minutes. The control mouse is kept in one chamber via foot shock boards (0.3 mAMPs) placed at the tube entrance. The foot shock boards were manually activated, as to avoid shocking the test mice. On day five, test mice are given the opposite injection than day four and the process is repeated. A different set of BALB/c mice were used on day five, preventing test mice from encountering the same control mouse twice.
Figure 2. Experimental Design. Full, week by week timeline of experiment. Behavioral assay in week 6 is detailed day by day.
Figure 6. Images of the nucleus accumbens (NAc) in brain J taken with a fluorescent microscope. A. The right side of the brain with mCherry expressed in the shell of the NAc. B. The right side of the brain with GFP expressed, primarily surrounding/being diluted by the expressed mCherry. C. The left side of the brain with mCherry expressed in the center of the NAc. D. The left side of the brain with GFP expressed around the center of the NAc.
DISCUSSION
Silencing this specific projection from the BNST resulted in different behavioral trends than silencing the entire BNST. Overall, there was not a significant increase in social aversion in socially isolated or group housed mice when the BNST to NAc projecting neurons were silenced. This is likely because mice appeared to make quick decisions before entering the social chamber. So, the contribution of social hesitancy scores pulled the overall social aversion score down (indicating less of the social anxiety phenotype). Once in the social chamber, isolated mice then exhibited an increase in anxious behaviors. These mixed results of social aversion, decreased hesitancy but increased freezing/reactivity, point to many possible explanations that require further experimentation. The first would be that the specific social motivation and social fear neural pathways involve overlapping populations of neurons, likely including the targeted projections silenced. Both social motivation increased (as hesitancy decreased) and social fear increased therefore the same neurons may be involved in controlling both of those behaviors. In this explanation, the targeted neurons would be intermediate participants in multiple, prominent social behavior inducing neural pathways.
Another likely explanation would be that there is one neuron or a subset of neurons, likely those silenced, projecting to multiple other neurons that belong to different pathways, some of which control social fear and some of which control social hesitancy. In this explanation, the targeted neuron would be near the beginning of multiple social pathways. It is possible the silenced neuron inhibits a neuron in the social hesitancy pathway while exciting a neuron in the social fear pathway, or potentially even more specific behavioral pathways.
It is also possible that the targeted projections control a behavioral readout, rather than the emotional state. Different emotions can lead to different behaviors. While mice may be experiencing anxiety, which involves the entire BNST, these specific projections may control behaviors from the emotional state. There could be distinct subpopulations of neurons that control each behavioral action and its associated motor output, rather than contribute to an overall emotional state. If this were the case then the results would best be interpreted as just an output of behavior rather than contributing to the social anxiety phenotype, but the presence of multiple social behaviors is indicative of the social anxiety phenotype. None of these explanations are exclusive, and all could be true.
Previous literature has shown an increase in social motivation when the entire BNST was silenced, which is contrary to previous BNST silencing work we have done (Emmons et al., 2021). But, this is not entirely contrary to the results of this experiment. This experiment supports both an increase in social motivation and an increase in social anxiety. As there has yet to be a consistent conclusion drawn on the effects of silencing BNST neurons on social behavior, it is likely that neurons in the BNST control many differing social and fear behaviors. Silencing the entire BNST doesn’t always lead to simply more or less anxiety, because the neurons participate in more complex, sometimes contradicting pathways. For example, the BNST neurons targeted in this study displayed an involvement in hesitancy and fear, but appeared to inhibit one and amplify the other.
The scope of this study includes social anxiety born from social isolation as well as social anxiety due to the presence of a stranger. It only includes experimentation in which the targeted neurons were silenced, activation was done in a separate study. This research was limited by a sample size of 24 mice, which may have prevented the results from reaching significance and affected the generalizability of the results. There are also animal model constraints to consider. The findings are based on C57BL/6 mice, which may not fully translate to human social behavior and psychiatric conditions. The experiment was conducted within a restricted period (10 weeks), limiting long-term behavioral and neurobiological observations. Variability in viral expression efficiency and potential off-target effects of chemogenetic manipulation (DREADDs) may influence results, though brains of all experimental mice were imaged and those that did not express proper viral placement were excluded from behavioral data analysis. While SAUSI provides valuable insight into social interactions, it may not capture all aspects of social cognition or anxiety-related behaviors in mice, repeated experimentation using miniature, head mounted microscopes (miniScope) has already been underway to capture more real-time neural activity.
The results of this project not only spark further research questions and experimental plans, but they carry significance in the fields of psychiatric and therapeutic research. The BNST to NAc projecting neurons exhibit a contribution to aspects of social behavior and anxiety, though these contributions may not be uniform. Those who suffer from mental illnesses and disorders, especially those that experience social aversion, don’t always experience the same struggles. Different individuals may need different treatments, based on their experiences. Being able to identify the specific effects of silencing the targeted neurons allowed for specific results and could lead to the development of specific treatments. Maybe an individual struggles with social motivation, but once they are socializing, they don’t feel anxious. Or an individual is eager to socialize, but spends the whole event hiding from others. Understanding their neural activity, aids professionals in treating their symptoms. Further manipulation of BNST and NAc neurons is necessary for understanding social aversion disorders.
Bibliography
Accumbens Core and Shell in Incentive-Cue Responding and Behavioral Inhibition. The Journal of Neuroscience, 31(18), 6820–6830. https://doi.org/10.1523/JNEUROSCI.6491-10.2011
BioRender. (n.d.). Retrieved February 25, 2025, from https://app.biorender.com/illustrations/67be88e3f29d4d9e4ccc998a
C57BL/6 Mice. (n.d.). Charles River. Retrieved February 25, 2025, from https://www.criver.com/products-services/find-model/c57bl6-mouse
CDC. (2024, July 19). Data and Statistics on Autism Spectrum Disorder. Autism Spectrum Disorder (ASD). https://www.cdc.gov/autism/data-research/index.html
Daniel, S. E., & Rainnie, D. G. (2016). Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(1), 103–125. https://doi.org/10.1038/npp.2015.178
Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2010). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35(1), 105–135. https://doi.org/10.1038/npp.2009.109
Emmons, R., Sadok, T., Rovero, N. G., Belnap, M. A., Henderson, H. J. M., Quan, A. J., Del Toro, N. J., & Halladay, L. R. (2021). Chemogenetic manipulation of the bed nucleus of the stria terminalis counteracts social behavioral deficits induced by early life stress in C57BL/6J mice. Journal of Neuroscience Research, 99(1), 90–109. https://doi.org/10.1002/jnr.24644
Fox, M. E., Figueiredo, A., Menken, M. S., & Lobo, M. K. (2020). Dendritic spine density is increased on nucleus accumbens D2 neurons after chronic social defeat. Scientific Reports, 10(1), 12393. https://doi.org/10.1038/s41598-020-69339-7
Goodwin, R. D., Weinberger, A. H., Kim, J. H., Wu, M., & Galea, S. (2020). Trends in anxiety among adults in the United States, 2008-2018: Rapid increases among young adults. Journal of Psychiatric Research, 130, 441–446. https://doi.org/10.1016/j.jpsychires.2020.08.014
Grammer, J., Valles, R., Bowles, A., & Zelikowsky, M. (2024). SAUSI: An integrative assay for measuring social aversion and motivation (p. 2024.05.13.594023). bioRxiv. https://doi.org/10.1101/2024.05.13.594023
Holt-Lunstad, J. (2024). Social connection as a critical factor for mental and physical health: Evidence, trends, challenges, and future implications. World Psychiatry, 23(3), 312–332. https://doi.org/10.1002/wps.21224
Holt-Lunstad, J., Smith, T. B., Baker, M., Harris, T., & Stephenson, D. (2015). Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 10(2), 227–237. https://doi.org/10.1177/1745691614568352
Ike, K. G. O., de Boer, S. F., Buwalda, B., & Kas, M. J. H. (2020). Social withdrawal: An initially adaptive behavior that becomes maladaptive when expressed excessively. Neuroscience & Biobehavioral Reviews, 116, 251–267. https://doi.org/10.1016/j.neubiorev.2020.06.030
jhalper. (2025, February 26). The Rise of Social Anxiety Disorder in America. The Blueprint. https://www.bluekc.com/blueprint/healthier-living/the-rise-of-social-anxiety-disorder-in-america/
Kai, N., Nishizawa, K., Tsutsui, Y., Ueda, S., & Kobayashi, K. (2015). Differential roles of dopamine D1 and D2 receptor-containing neurons of the nucleus accumbens shell in behavioral sensitization. Journal of Neurochemistry, 135(6), 1232–1241. https://doi.org/10.1111/jnc.13380
Lebow, M. A., & Chen, A. (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. https://doi.org/10.1038/mp.2016.1
Loneliness and Social Isolation as Risk Factors for Mortality: A Meta-Analytic Review—Julianne Holt-Lunstad, Timothy B. Smith, Mark Baker, Tyler Harris, David Stephenson, 2015. (n.d.). Retrieved March 4, 2025, from https://journals.sagepub.com/doi/full/10.1177/1745691614568352
Loneliness in America: How the Pandemic Has Deepened an Epidemic of Loneliness. (2025, January 23). Making Caring Common. https://mcc.gse.harvard.edu/reports/loneliness-in-america
Ma, L., Chen, W., Yu, D., & Han, Y. (2020). Brain-Wide Mapping of Afferent Inputs to Accumbens Nucleus Core Subdomains and Accumbens Nucleus Subnuclei. Frontiers in Systems Neuroscience, 14, 15. https://doi.org/10.3389/fnsys.2020.00015
Mathew, S. J., Coplan, J. D., & Gorman, J. M. (2001). Neurobiological mechanisms of social anxiety disorder. The American Journal of Psychiatry, 158(10), 1558– 1567. https://doi.org/10.1176/appi.ajp.158.10.1558
Netser, S., Meyer, A., Magalnik, H., Zylbertal, A., de la Zerda, S. H., Briller, M., Bizer, A., Grinevich, V., & Wagner, S. (2020). Distinct dynamics of social motivation drive differential social behavior in laboratory rat and mouse strains. Nature Communications, 11(1), 5908. https://doi.org/10.1038/s41467-020-19569-0
Nock, M. K., Hwang, I., Sampson, N., Kessler, R. C., Angermeyer, M., Beautrais, A., Borges, G., Bromet, E., Bruffaerts, R., De Girolamo, G., De Graaf, R., Florescu, S., Gureje, O., Haro, J. M., Hu, C., Huang, Y., Karam, E. G., Kawakami, N., Kovess, V., … Williams, D. R. (2009). Cross-National Analysis of the Associations among Mental Disorders and Suicidal Behavior: Findings from the WHO World Mental Health Surveys. PLoS Medicine, 6(8), e1000123. https://doi.org/10.1371/journal.pmed.1000123
Reite, M., & Field, T. (Eds.). (1985). BEHAVIORAL BIOLOGY: AN INTERNATIONAL SERIES. In The Psychobiology of Attachment and Separation (p. ii). Academic Press. https://doi.org/10.1016/B978-0-12-586780-1.50001-X
Richey, J. A., Rittenberg, A., Hughes, L., Damiano, C. R., Sabatino, A., Miller, S., Hanna, E., Bodfish, J. W., & Dichter, G. S. (2014). Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Social Cognitive and Affective Neuroscience, 9(3), 367–377. https://doi.org/10.1093/scan/nss146
Sareen, J. (2014). Posttraumatic Stress Disorder in Adults: Impact, Comorbidity, Risk Factors, and Treatment. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 59(9), 460–467. SAUSI: an integrative assay for measuring social aversion and motivation | bioRxiv. (n.d.). Retrieved February 25, 2025, from https://www.biorxiv.org/content/10.1101/2024.05.13.594023v2.full
Social Anxiety Disorder—National Institute of Mental Health (NIMH). (n.d.). Retrieved March 4, 2025, from https://www.nimh.nih.gov/health/statistics/social-anxiety-disorder
Trezza, V., Damsteegt, R., Achterberg, E. J. M., & Vanderschuren, L. J. M. J. (2011). Nucleus Accumbens μ-Opioid Receptors Mediate Social Reward. Journal of Neuroscience, 31(17), 6362–6370. https://doi.org/10.1523/JNEUROSCI.5492-10.2011
van de Poll, Y., Cras, Y., & Ellender, T. J. (2023). The neurophysiological basis of stress and anxiety—Comparing neuronal diversity in the bed nucleus of the stria terminalis (BNST) across species. Frontiers in Cellular Neuroscience, 17, 1225758. https://doi.org/10.3389/fncel.2023.1225758
Walker, D. L., Toufexis, D. J., & Davis, M. (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463(1–3), 199–216. https://doi.org/10.1016/s0014-2999(03)01282-2
Walsh, J. J., Christoffel, D. J., Wu, X., Pomrenze, M. B., & Malenka, R. C. (2021). Dissecting neural mechanisms of prosocial behaviors. Current Opinion in Neurobiology, 68, 9–14. https://doi.org/10.1016/j.conb.2020.11.006
Wu, Y. E., & Hong, W. (2022). Neural basis of prosocial behavior. Trends in Neurosciences, 45(10), 749–762. https://doi.org/10.1016/j.tins.2022.06.008
Xiao, Q., Zhou, X., Wei, P., Xie, L., Han, Y., Wang, J., Cai, A., Xu, F., Tu, J., & Wang, L. (2021). A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety. Molecular Psychiatry, 26(9), 4719–4741. https://doi.org/10.1038/s41380-020-0816

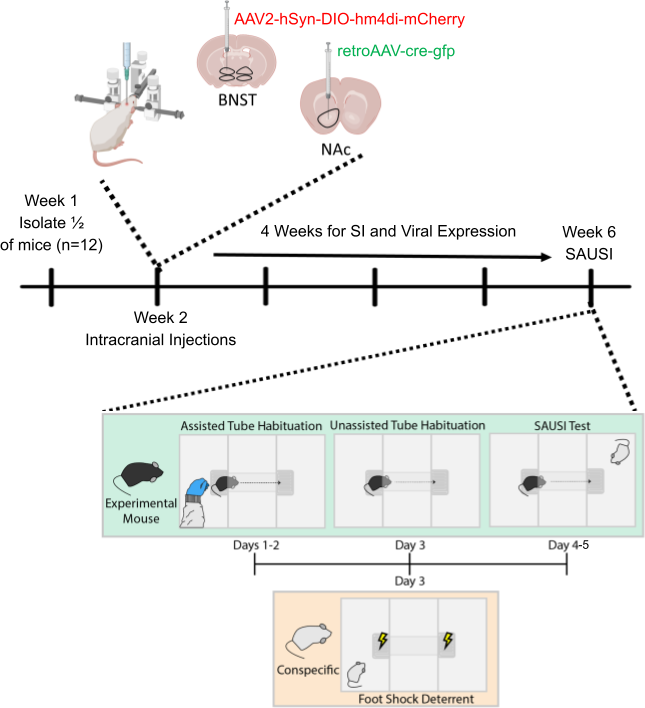
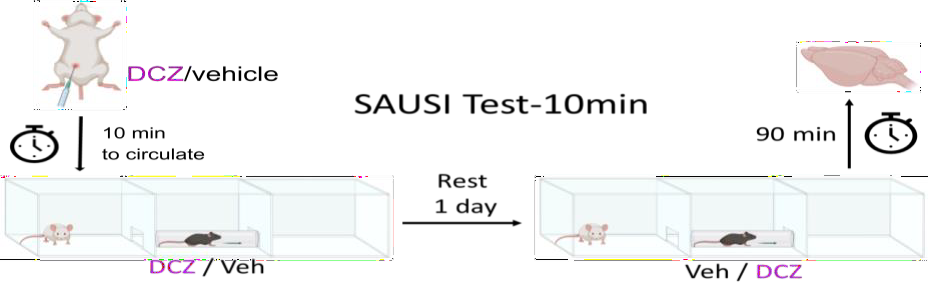
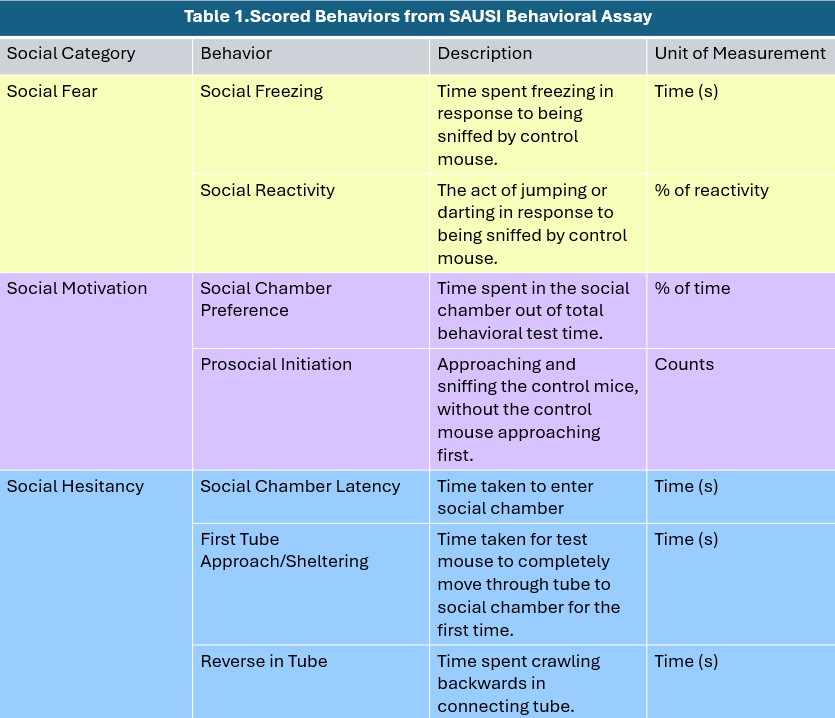
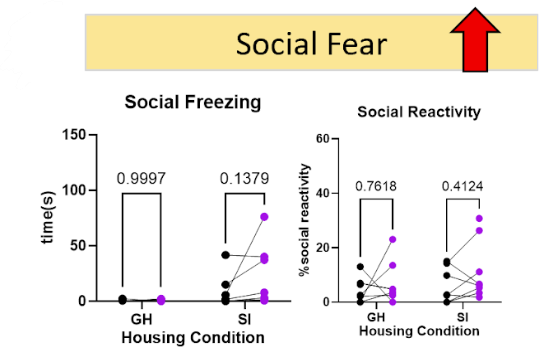
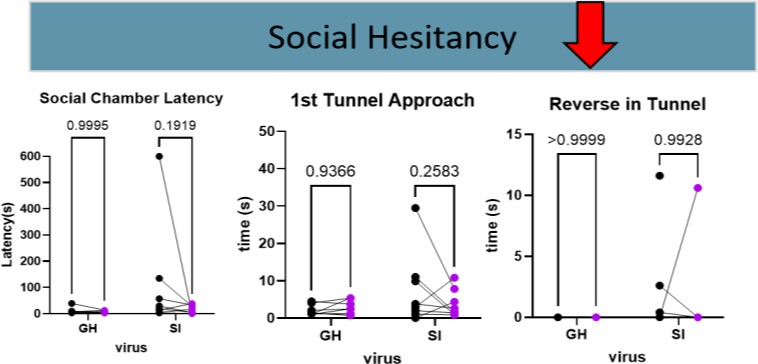

 Figure 6B.
Figure 6B.