Spencer Fox Eccles School of Medicine
73 Long-Chain Polyunsaturated Fatty Acid Surge and Altered PPARγ Isoform Ratios Precede Alveolar Formation in the Developing Rat Lung
Daniel Wray; Lisa Joss-Moore; Wesley Chidester; and Sofia Leveratto
Faculty Mentor: Lisa Joss-Moore (Pediatrics, University of Utah)
Background
The lung disease bronchopulmonary dysplasia (BPD) is prevalent in preterm neonates and is characterized by impaired alveolar development (1). Alveolar development depends on adequate nutrient provision at critical developmental stages. Nutrients critical for alveolar formation include the long-chain polyunsaturated fatty acids (LCPUFAs), specifically, the omega-3 docosahexaenoic acid (DHA) and the omega-6 arachidonic acid (ARA) (2). While the importance of essential fatty acids in lung development has been recognized for over a decade, recent clinical trials highlight potential negative consequences of postnatal supplementation with isolated DHA. In the N3RO trial, neonates received enteral DHA supplementation (3), and the MOBYDick Trial, neonates received maternal breast milk from mothers supplemented with DHA (4). Both trials demonstrated a potential negative impact of supplemental DHA on the development of BPD, and both trials studied neonates born at less than 29 weeks of gestation.
An important consideration is the timing of lung development. The last two stages of lung development are the saccular and alveolar sages. In the saccular stage, the lung is characterized by thick cellular walls. As lung development continues, the saccular-to-alveolar transition takes place, where the lung is characterized by progressively thinner walls and smaller, more numerous alveoli with increased secondary septa. As the bulk formation of alveoli continues, the microvascular beds and alveolar refinement ensure a structure with appropriate capacity for gas exchange (Figure 1). In the human lung, the saccular-to-alveolar transition occurs late in gestation, and microvascular maturation and alveolar refinement continue postnatally. This is in contrast to the preterm human lung, which may be in the saccular stage at birth (5).
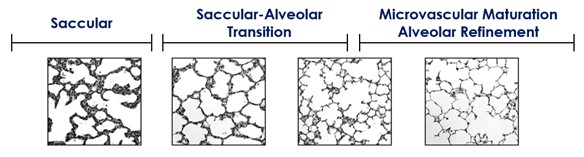
Figure 1. Gross lung structure during the developmental time periods. Showing development from saccular stage, through the transition and finally the alveolar stage.
Alveolar formation is in part mitigated by PPARγ, a lipid-responsive transcription factor. PPARγ is activated by a ligand, such as DHA. Activation of PPARγ results in its nuclear translocation, binding to a response element in a target gene, and subsequent expression of the target gene. PPARγ target genes important in the developing lung include Perilipin 2, also known as Adipose differentiation- related protein, which is particularly important for the epithelial-mesenchymal transition that drives lung development. As for many transcription factors, regulation of PPARγ activity is complex and involves many additional aspects, including alternative splicing (6).
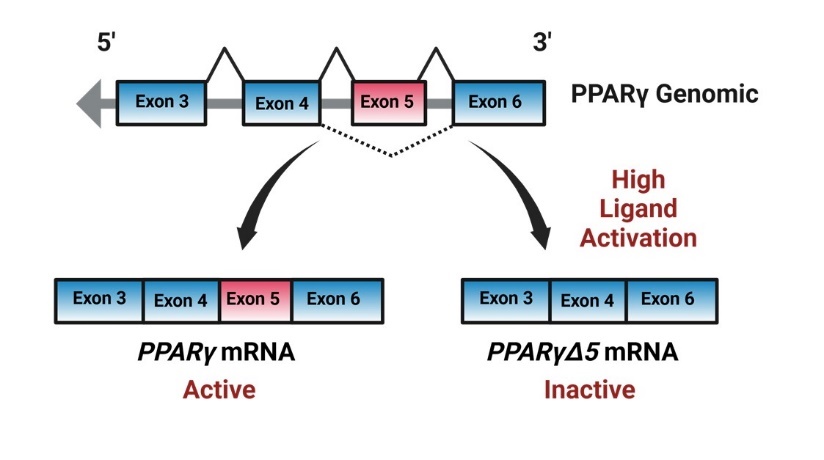
Figure 2. Graphics demonstrating PPARγ alternative splicing, genomic PPARγ normally expresses exons 4,5 and 6 in the fully active mRNA variant. However, with high ligand activation, genomic PPARγ will alternatively splice excluding exon 5 creating the pparγΔ5 mRNA variant.
Standard transcription of the gene produces a transcript that includes exons 4, 5, and 6. However, under conditions of high ligand activation, for example by DHA, PPARγ undergoes alternative splicing of exon 5. The transcript lacking exon 5 is known as pparγΔ5, and it codes for a dominant- negative or inactive PPARγ protein isoform (Figure 2). While the standard or full-length variant codes for the active protein, the ratio of PPARγ to PPARγΔ5 reflects PPARγ’s activity potential. While we’ve shown that pparγΔ5 is expressed in the rat lung (7), its timing and relationship to lung fatty acid levels remain unclear. We hypothesize that essential fatty acids will vary across the saccular-to-alveolar transition and correlate with levels of pparγ/pparγΔ5, or the activity potential of PPARγ in the rat lung.
Methods
For this study, we used lungs from Sprague Dawley rats at different postnatal ages. All animal procedures were reviewed and approved by the University of Utah Animal Care and Use Committee, following the NIH Guidelines for the Care and Use of Laboratory Animals. The rat lung undergoes the same developmental stages as the human lung, except with different timing. In the rat, the lung is in the saccular stage at term birth, with the saccular-to-alveolar transition and subsequent alveolar refinement occurring postnatally, similar to that of the preterm human lung. In this study, we examined rat lungs at postnatal day of life 0 and day 2, both representing the saccular stage, day of life 7 and 12 during the saccular-alveolar transition, and day of life 16 and 21 during the alveolar refinement stage. D0 rat pups were delivered by C-section at term, and all postnatal ages were delivered vaginally, with litters culled to 8 pups to ensure constant postnatal nutrition.
Targeted lipidomics, performed by the University of Utah Metabolomics Core Facility, was used to measure DHA and ARA in the lung. Real-time RT-PCR was used to measure pparγ and pparγΔ5 mRNA levels. We used an assay-on-demand primer/probe set for pparγ, which covers the exon boundary between exon 5 and 6 (Rn00440945_m1, ThermoFisher Scientific, Waltham, MA, USA). To measure levels of pparγΔ5, we used a custom primer/probe set spanning the exon 4–6 junction (forward, CGAGAAGGAGAAGCTGTTGG; reverse, GCGGTTGATTTGTCTGTTGT; probe, CCCTGGCAAAGCATTTGTAT). For all measures, the comparative CT method was used, with GADPH as an internal control. Statistical differences were determined using ANOVA with post-hoc least squares analysis.
Results
We first determined the concentration of the fatty acids in the lung over the developmental timeline. Data were analyzed by sex, and there were no differences between male and female lungs, so sexes are combined. Levels of DHA increase over the saccular stage and drop significantly during the saccular- to-alveolar transition, especially at day 12, rebounding somewhat during alveolar refinement (Figure 3).
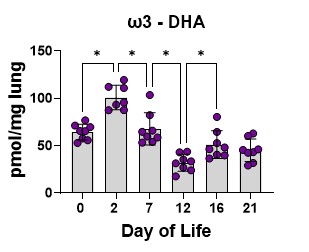
Figure 3. Lung DHA levels. Data are pmol DHA/mg of lung tissue at the developmental timelines studied. The bars are the mean, and the error bars are the standard deviation. The circles represent lung tissue samples from individual rats. Asterisks represent a significant difference compared to the adjacent time point.
ARA levels show a similar pattern to DHA, again with a decrease at day 12 toward the end of the transition and a slight rebound after (Figure 4).
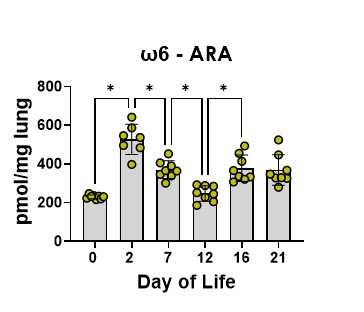
Figure 4. Lung ARA levels. Data are pmol DHA/mg of lung tissue at the developmental timelines studied. The bars are the mean, and the error bars are the standard deviation. The circles represent lung tissue samples from individual rats. Asterisks represent a significant difference compared to the adjacent time point.
We then determined the relative amounts of DHA to ARA across the developmental timeline as this ratio is important for many cellular functions. The DHA to ARA ration decreases steadily throughout alveolar formation, again showing a significant dip at day 12 toward the end of the transition (Figure 5).
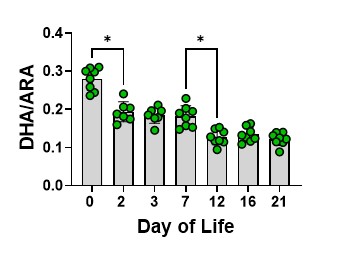
Figure 5. The DHA to ARA ratio across the developmental timelines studied. The bars are the mean, and the error bars are the standard deviation. The circles represent lung tissue samples from individual rats. Asterisks represent a significant difference compared to the adjacent time point.
We then determined how these fatty acid changes related to the expression of PPARγ and PPARγΔ5, particularly how they affected the ratio of PPARγ to PPARγΔ5, or the activity potential of PPARγ. The activity potential of PPARγ decreases before the saccular-to-alveolar transition and then increases during the transition and later alveolar refinement (Figure 6).
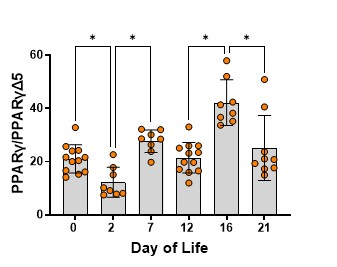
Figure 6. The ratio of PPARγ to PPARγΔ5 across the developmental timelines studied. The bars are the mean, and the error bars are the standard deviation. The circles represent lung tissue samples from individual rats. Asterisks represent a significant difference compared to the adjacent time point.
Finally, we assessed the correlation between the PPARγ activity potential and lung DHA as well as DHA/ARA. Both DHA and the DHA/ARA are significantly correlated with the PPARγ activity potential (Figure 7a, 7b).
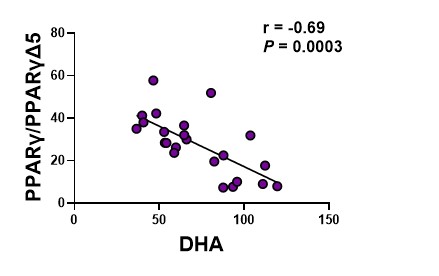
Figure 7a. the ratio of PPARγ to PPARγΔ5 relative to levels of DHA. The circles represent lung tissue samples from individual rats.
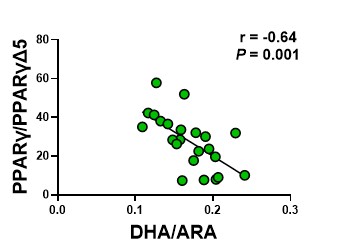
Figure 7b. the ratio of PPARγ to PPARγΔ5 relative to the DHA to ARA ratio. The circles represent lung tissue samples from individual rats.
Summary and Conclusion
Here we showed that lung levels of DHA and ARA are high in the saccular stage of lung development and decrease through alveolar transition. We also showed that the PPARγ activity potential shows a reciprocal pattern with the DHA/ARA ratio, being low in the saccular stage and increasing through alveolar transition. Lastly, lung DHA and the DHA/ARA ratio are significantly negatively correlated with PPARγ activity potential in all lung stages examined. We conclude that DHA and ARA levels and the PPARγ activity potential fluctuate in a coordinated manner during critical stages of alveolar formation. Given that PPARγ activity is vital to appropriate saccular-to-alveolar transition in the lung, and that DHA acts to reduce the PPARγ activity potential, we speculate that the normal decrease in lung DHA during the saccular-to-alveolar transition may facilitate PPARγ activity. We further speculate that isolated DHA supplementation during the transition may result in reduced PPARγ activity by increasing the production of PPARγΔ5, thus impairing alveolar formation and increasing BPD risk.
Bibliography
Joss-Moore LA, Lane RH, Albertine KH. Epigenetic contributions to the developmental origins of adult lung disease. Biochem Cell Biol. 2015;93(2):119-127. doi:10.1139/bcb-2014- 0093
Wiedmeier JE, Joss-Moore LA, Lane RH, Neu J. Early postnatal nutrition and programming of the preterm neonate. Nutr Rev. 2011;69(2):76-82. doi:10.1111/j.1753-4887.2010.00370.x
Collins, C.T., et al., Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. N Engl J Med, 2017. 376(13): p. 1245-1255.
Marc, I., et al., Effect of Maternal Docosahexaenoic Acid Supplementation on Bronchopulmonary Dysplasia-Free Survival in Breastfed Preterm Infants: A Randomized Clinical Trial. JAMA., 2020. 324(2): p. 157-167. doi:10.1001/jama.2020.8896
.DiFiore JW, Wilson JM. Lung development. Semin Pediatr Surg. 1994;3(4):221-232.
Aprile, M., et al., PPARγΔ5, a Naturally Occurring Dominant-Negative Splice Isoform, Impairs PPARγ Function and Adipocyte Differentiation. Cell Rep., 2018. 25(6): p. 1577- 1592.e6. doi: 10.1016/j.celrep.2018.10.035.
Cohen AJ, Chidester WR, Wray DT, et al. Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity. Biomolecules. 2025;15(4):551. Published 2025 Apr 9. doi:10.3390/biom15040551

