84 Onset and Offset Dynamics in Animal Models of Epilepsy: Response to Valproic Acid and Phenobarbital
Kishore Jay
Faculty Mentor: Karen Wilcox (Biomedical Engineering, University of Utah)
Epilepsy is a neurological disorder that affects more than 3 million people nationwide and is characterized by sudden and recurrent seizures [1]. Epilepsy is commonly diagnosed using electroencephalography (EEG) to identify unusual electrical activity in the brain, characteristic of epilepsy syndrome. Current epilepsy treatments are administered based on symptom characteristics and EEG pattern analysis [2].
However, epilepsy treatment is complicated because seizure prognosis is highly variable. Additionally, similar clinical therapies produce different patient outcomes [3]. The lack of clarity in seizure characterization and the resulting ineffective treatment options stem from the limitations of current clinical classification systems. Saggio et al. [4] identified novel methods of classification, applying bifurcation theory to EEG shapes to identify “dynamotypes,” as applied specifically to epileptic seizures. Bifurcations define the transitions in the underlying behavioral dynamics that can be observed by changes in wave statistics, including onset and offset of oscillations, relevant to electrical wave propagation in seizures. The dynamotype describes the EEG pattern for the first 5 seconds (onset) and last 5 seconds (offset) of a seizure.
This project aids the identification and classification of these seizure dynamotypes recorded through hippocampal EEG measured in the temporal lobe epilepsy (TLE) mouse model, with the hypothesis that dynamotypes change over the course of seizure evolution and are affected by the administration of anti-seizure medications (ASM). Categorization of these dynamotypes would improve the understanding of seizure properties by tracking the evolution of seizure conditions. The study design uses previously collected data to investigate how dynamotypes change over time and whether dynamotypes can predict drug response. The EEG from these mice collected five seconds of seizure onset and five seconds of seizure offset data. These data were analyzed via a machine learning algorithm as well as two trained researchers to categorize the seizures into the specific onset and offset dynamotypes.
As previously stated, the categorization of these seizure characteristics can improve our understanding of seizure properties. By allowing capabilities to track the evolution of a patient’s seizure conditions, administration of anti-epileptic drugs will be made more purposeful and effective. This method of seizure classification could be the first step in the pathway from EEG to diagnosis to therapy, as the series of correlative observations are being performed in an in vivo epilepsy model.
Background
Within the scope of this project, there are three onset dynamotypes, identified within the first five seconds of the seizure EEG: Supercritical Hopf (SupH), characterized by increasing amplitude of oscillations, Saddle-Node Invariant Circle (SNIC), characterized by increasing spike frequency, and Sub-critical Hopf (SubH), characterized by arbitrary patterns in amplitude and/or frequency. Identified in the last five seconds before seizure termination are the following three offset dynamotypes: SupH for decreasing amplitude of oscillations, SNIC for decreasing spike frequency, and SubH, characterized by arbitrary patterns in amplitude and/or frequency, as shown in Figure 1.
In a published study by West et al. 2019 [5], a group of mice were administered kainic acid and observed to ensure the onset of status epilepticus (i.e., the occurrence of seizures that last for longer than five minutes as well as multiple seizures that occur consecutively within a short period) and regularity of seizures. The study subjects were monitored under 24/7 video and EEG recording. This provided the preliminary data sets to test our hypothesis that dynamotypes, based on EEG, change over a set period. The study quantified the detected seizures following the Racine scale, which ranks the strength of seizures from 1-5 based on observable behaviors, such as facial movements, head nodding, and forelimb clonus [6]. The Racine scale is commonly used to assess the severity of seizures in experimental models of epilepsy. The scale serves to standardize the evaluation of seizure severity, facilitating consistent comparison across studies and aiding in the assessment of therapeutic interventions in epilepsy research.
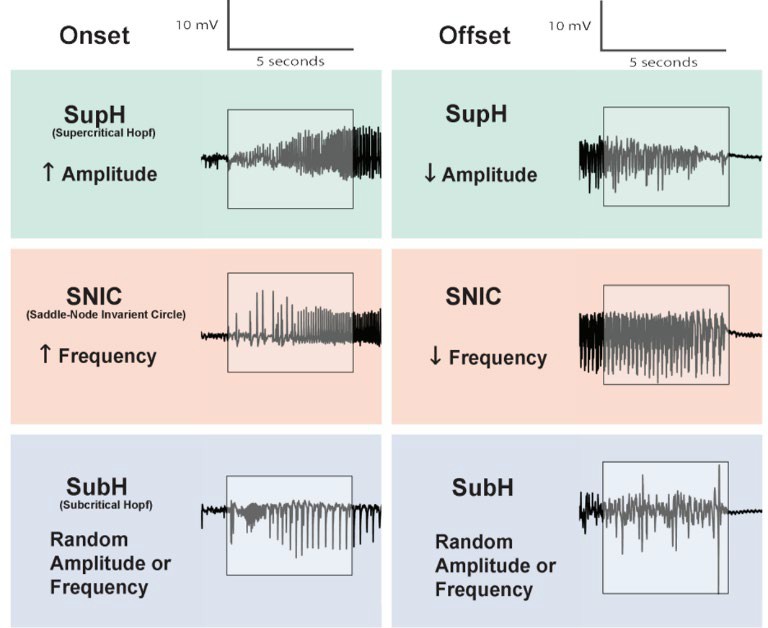
Figure 1. Onset and offset EEG dynamotypes of seizures, within the scope of this study.
METHODS
Animal Model and Seizure Monitoring
To investigate seizure frequency and severity in the interamygdala kainic acid (IAK) mouse model of temporal lobe epilepsy (TLE), mice were continuously monitored using both video recordings and electroencephalography (EEG). This allowed for the precise detection of seizure events and their characteristics over an extended observation period.
Study Design and Drug Administration
A blinded, double-crossover study was conducted to assess the effects of two anticonvulsant drugs, valproic acid (VPA) and phenobarbital (PB), on seizure activity. Valproic acid has a complex and not yet fully understood mechanism of action, though it is believed to work by inhibiting both voltage-gated sodium channels and gamma-aminobutyric acid (GABA) receptors [7]. In contrast, phenobarbital primarily acts by inhibiting GABA receptors [8]. Comparing the effects of these two drugs could reveal whether seizure dynamotypes are more influenced by sodium channel inhibition or GABA receptor modulation. The study was structured as follows:
- Baseline Monitoring: All mice were first subjected to a seven-day baseline period, during which their EEG signals were recorded without any drug intervention.
- Initial Drug Administration: Following baseline recording, half of the mice were administered either VPA or PB, while the other half received a saline solution as a control. EEG monitoring continued for five days post- administration to assess any immediate effects on seizure frequency and severity.
- Washout Period: To eliminate any residual effects of the administered compounds, a two-day drug washout period was implemented, during which no treatments were given.
- Crossover Phase: After the washout period, the treatment groups were reversed. The mice that initially received the drug were now administered saline, while the control group received the drug. EEG monitoring continued for an additional five days to evaluate changes in seizure characteristics under the new conditions.
The blinded nature of the study ensured that researchers assessing seizure activity were unaware of whether a given mouse had received the drug or saline, preventing potential bias in data interpretation.
Seizure Classification and Dynamotyping
To systematically analyze seizure activity, seizures were classified based on their onset and offset dynamotypes—patterns that characterize the beginning and end of each seizure event. This process, referred to as dynamotyping, was performed by three independent graders: two human researchers and a machine learning algorithm trained for seizure classification. The grading was performed by the human graders using MATLAB software.
To enhance accuracy and consistency in dynamotype classification, a consensus-based approach was used. A seizure onset or offset was only assigned to a dynamotype if at least two of the three graders agreed on the classification. If no agreement was reached, the seizure event was excluded from the dataset.
Data Analysis
The collected EEG data were analyzed to determine the frequency and duration of seizures under different treatment conditions. Statistical analyses were performed to assess the significance of observed changes in seizure patterns, including shifts in onset and offset dynamotypes across baseline, drug-dosed, and crossover conditions.
Results
To investigate seizure frequency and severity in the IAK mouse model of TLE, mice were continuously monitored via video and EEG. A blinded, double-crossover study was conducted to assess the effects of valproic acid and phenobarbital. Following a seven-day baseline EEG recording, mice were alternately administered either the drug or saline, followed by a washout period and a crossover of treatments. The seizure onset and offset classification was conducted through a “dynamotyping” approach that involved two human graders and a machine learning algorithm, with consensus among at least two graders required for inclusion in the final dataset. The outcomes of this study are presented below.
90-day Murine Study
In the first 30 days of the 90-day murine study, seizure onset dynamotypes were predominantly arbitrary-waveforms (SubH), accounting for 78% of cases. However, as shown in Figure 2, this percentage gradually decreased over time, dropping to 59% in the middle 30 days and 49% in the final 30 days.
A similar trend was observed in offset dynamotypes. During the first 30 days, SubH was the dominant classification (86%). By the final 30 days, however, the prevalence of SNIC and SupH increased, while SubH showed a significant reduction over time (Kruskal-Wallis Test, p = 0.058).
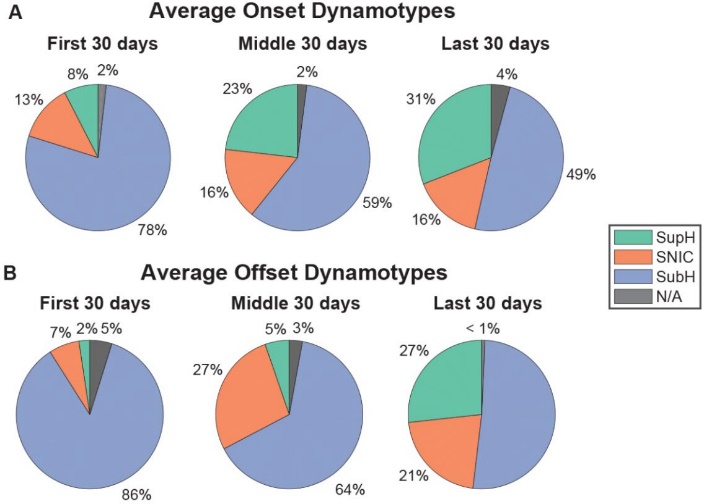
Figure 2. Ratios of onset and offset dynamotypes, recorded in the first, middle, and last 30 days of the 90-day observation period. Notice the downward trend of SubH in both onset and offset cases.
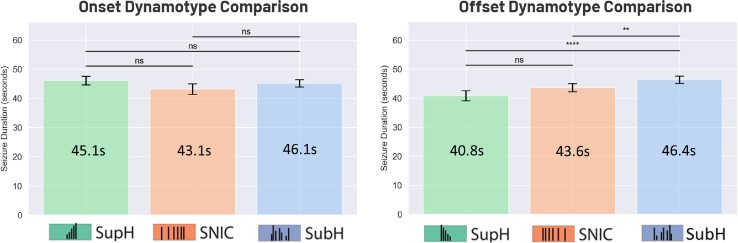
The average seizure duration was analyzed based on onset dynamotypes (SupH, SNIC, and SubH) and then compared. A similar analysis was conducted for offset dynamotypes. As shown in Figure 3, the results indicate that onset dynamotype did not significantly impact seizure duration. However, seizures with an amplitude-based (SupH) or frequency-based (SNIC) offset were significantly shorter than those with an arbitrary-waveform (SubH) offset (Linear Mixed Model, p = 0.003, p = 0.0006). This suggests that seizures with a more structured offset pattern terminate more quickly than those with a randomized offset.
Figure 3. Durations of seizures matched with onset dynamotypes (left), and durations of seizures matched with offset dynamotypes (right).
Drug Cohort Study
As shown in Figure 4, dosing with valproic acid (VPA) did not result in changes to seizure onset dynamotypes. In both baseline and dosed cohorts, the SubH dynamotype remained predominant, accounting for 77% and 72% of cases, respectively. However, VPA had a notable effect on offset dynamotypes. The percentage of SNIC offsets nearly doubled, increasing from 26% at baseline to 53% following drug administration. Phenobarbital, however, did not produce similar effects. Both onset and offset dynamotypes remained consistent between baseline and drug-dosed conditions, with no statistically significant differences observed.
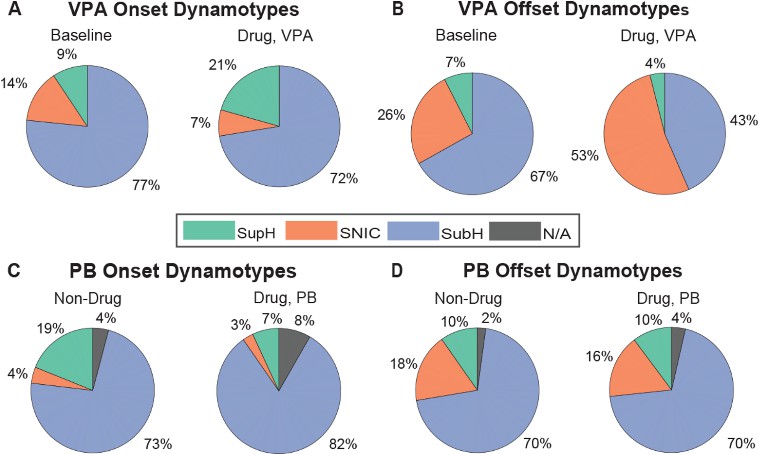
Figure 4. Ratios of onset and offset dynamotypes before and after dosing with anti-seizure medications. A. There was no significant change to onset dynamotype after VPA dosing. B. SubH offset significantly decreased between baseline and drug dosing periods, with a corresponding increase in SNIC offset (Kruskal-Wallis Test, p = 0.04). C and D. There was no significant difference between the drug and baseline period for both onset and offset dynamotype for PB.
Discussion
This study evaluated the hypothesis that seizure dynamotypes evolve over time and may predict responses to anti-seizure medications (ASMs). Characterized by EEG analysis in a temporal lobe epilepsy (TLE) mouse model, dynamotypes are certain measurable ways that a seizure signal starts and stops. By analyzing the dynamotype profiles of seizures at onset and offset, this research provides a foundation for improving diagnostic and therapeutic approaches for epilepsy.
The findings demonstrate a clear temporal evolution of dynamotypes. Arbitrary-waveform (SubH) onset and offset dynamotypes dominated during the first 30 days, representing 78% and 86% of seizures, respectively. However, the prevalence of SubH decreased significantly over the 90-day period, with concurrent increases in frequency-based (SNIC) and amplitude-based (SupH) offset dynamotypes. These trends suggest that the dynamotype framework can capture subtle pathological shifts in seizure dynamics over time. The observed reduction in SubH dynamotypes would indicate an adaptation or progression in the underlying biochemical interactions, warranting further exploration into the mechanisms driving these transitions.
These results align with studies that have examined bifurcation theory in seizure dynamics, which predict transitions between different seizure termination patterns based on neuronal excitability and inhibition [9]. Seizures terminating through frequency-based (SNIC) and amplitude-based (SupH) mechanisms may involve more stable inhibitory processes, whereas SubH patterns indicate greater variability and incomplete neuronal stabilization. The gradual decline in SubH offsets observed in this study supports the hypothesis that seizure networks undergo adaptive reconfiguration over time, potentially as a compensatory response to chronic seizure activity.
While these findings support existing bifurcation models, they also differ from other previous works. Some studies suggest that seizure offsets are stochastic and arbitrary and do not exhibit predictable transitions over time [10]. However, the results of this study challenge this notion by demonstrating a statistically significant shift toward structured offsets over 90 days, suggesting that these transitions are not merely random but reflect a more fundamental change in seizure network properties.
Furthermore, the observed reduction of SubH offsets raises important questions about whether long-term seizure activity itself alters network excitability in ways that favor structured termination patterns. Previous research in this area has shown that seizure-induced synaptic plasticity can promote specific network adaptations [11], but the role of dynamotype shifts in this process remains unexplored.
Seizures with SupH and SNIC offsets were found to be significantly shorter than SubH offsets, suggesting that seizures that have a stereotyped and structured offset pattern can terminate more efficiently than those with arbitrary patterns. This finding implies that specific physiological or network-level mechanisms contribute to a more rapid resolution of seizure activity when the offset follows a predictable amplitude- or frequency-based trajectory. One possible explanation is that structured offset patterns, such as SupH and SNIC, may indicate a more synchronized and coordinated neuronal inhibition process, facilitating faster seizure cessation. This aligns with previous studies on seizure suppression mechanisms, which have shown that network-wide inhibitory synchronization often coincides with more abrupt seizure termination [12]. In contrast, arbitrary- waveform (SubH) offsets might reflect a more disorganized or incomplete inhibitory response, leading to prolonged seizure durations. This finding is consistent with computational models that predict irregular seizure offsets occur when inhibitory mechanisms fail to fully engage [13], and this could have important implications for predicting seizure severity based on offset characteristics, potentially providing a biomarker for intractable epilepsy.
Analysis of drug effects on dynamotypes further highlights the potential clinical relevance of seizure profiling. VPA significantly increased the prevalence of SNIC offset dynamotypes, suggesting that it facilitates a transition toward structured seizure termination. This is consistent with studies showing that VPA enhances seizure inhibition through modulation of gamma-aminobutyric acid (GABA) receptors and sodium channel inhibition [14]. The findings of this study suggest that VPA’s effect may extend beyond seizure suppression to actively restructuring the seizure termination process.
Phenobarbital, on the other hand, showed no measurable effect on either onset or offset dynamotypes. While VPA enhances GABAergic inhibition as well as sodium channel inhibition, PB is known to only affect GABA receptors, and the finding that one significantly affects dynamotypes while the other doesn’t raises an interesting question: is the external manifestation of seizure dynamotypes dependent on the inhibitory post-synaptic potentials controlled by ion channels?
Future studies should explore the extent of the relationship between post-synaptic ion channels and the dynamotypes that arise from them. While this study provides valuable insights, certain limitations must be acknowledged. First, the analysis was conducted using data from a single animal model of TLE, which may limit generalizability to other epilepsy types or other species, including humans. Future work should expand dynamotyping to other models of genetic epilepsy, focal cortical dysplasia, and absence seizures to determine whether similar temporal transitions occur in those cases. Second, the study relied on EEG hippocampal recordings, potentially overlooking dynamotype variations in other brain areas.
Incorporating multi-region EEG or optogenetic techniques could provide a more complete picture of seizure network evolution. Finally, while our findings suggest that dynamotyping could inform ASM selection, prospective human studies are needed to determine whether pre-treatment dynamotype profiles can predict patient-specific drug responses. This could be especially relevant for drug- resistant epilepsy, where ASM prescription remains largely empirical.
However, despite these limitations, the results highlight the promise of dynamotyping as a novel approach to understanding and managing epilepsy. The identification of temporal changes in dynamotypes underscores their potential as indicators of epilepsy progression and therapeutic response approaches. If validated in human studies, dynamotyping could be integrated into clinical EEG analysis to provide personalized treatment recommendations. This would represent a major improvement over current seizure classification methods, which often rely on binary seizure/no seizure distinctions rather than detailed seizure evolution profiles. Furthermore, the significant shift in SNIC offset dynamotypes following VPA treatment suggests a biochemical link between ASMs and seizure termination dynamics, paving the way for more targeted therapeutic strategies. Future research should investigate whether specific molecular pathways, such as GABA receptor inhibition or post-synaptic ion channel mechanisms, drive these transitions.
Lastly, the application of machine learning to dynamotype classification could improve real-time seizure monitoring. Current seizure detection algorithms focus primarily on onset prediction, but integrating offset prediction and dynamotype evolution could lead to more effective, rapid therapeutic strategies. By bridging the gap between EEG characterization, seizure modeling, and therapeutic response prediction, dynamotyping has the potential to transform epilepsy care by providing a framework for precision medicine in neurology.
Reference
- S. L. Moshé, E. Perucca, P. Ryvlin, and T. Tomson, “Epilepsy: New advances,” The Lancet, vol. 385, no. 9971, pp. 884-898, Mar. 2015. DOI: https://doi.org/10.1016/s0140-6736(14)60456-6.
- E. C. Wirrell, L. Laux, E. Donner, et al., “Optimizing the Diagnosis and Management of Dravet Syndrome: Recommendations From a North American Consensus Panel,” Pediatric Neurology, vol. 68, pp. 18-34.e3, May 2017. [Online]. Available: https://www.pedneur.com/article/S0887- 8994(16)31037-2/fulltext.
- W. Löscher, H. Potschka, S. M. Sisodiya, and A. Vezzani, “Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options,” Pharmacological Reviews, vol. 72, no. 3, pp. 606-638, Jul. 2020. DOI: 10.1124/pr.120.019539.
- M. L. Saggio, D. Crisp, J. M. Scott, P. Karoly, L. Kuhlmann, and M. Nakatani, et al., “A taxonomy of seizure dynamotypes,” eLife, vol. 9, Jul. 2020, Art. no. e55632. DOI: https://doi.org/10.7554/eLife.55632.
- P. J. West, K. Thomson, P. Billingsley, T. Pruess, C. Rueda, and G. W. Saunders, et al., “Spontaneous recurrent seizures in an intra-amygdala kainate microinjection model of temporal lobe epilepsy are differentially sensitive to antiseizure drugs,” Experimental Neurology, vol. 349, Jan. 2022, Art. no. 113954. DOI: https://doi.org/10.1016/j.expneurol.2021.113954.
- J. R. Racine, “Modification of seizure activity by electrical stimulation: II. Motor Seizure,” Encephalography and Clinical Neurophysiology, vol. 32, 1972. DOI: https://doi.org/10.1016/0013-4694(72)90177-0.
- M. Rahman and H. Nguyen, “Valproic Acid (Divalproex Sodium),” PubMed, 2023. https://www.ncbi.nlm.nih.gov/books/NBK559112/.
- C. B. Lewis and N. Adams, “Phenobarbital,” PubMed, May 09, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532277/.
- V. K. Jirsa, W. C. Stacey, P. P. Quilichini, A. I. Ivanov, and C. Bernard, “On the nature of seizure dynamics,” Brain, vol. 137, no. 8, pp. 2210–2230, Aug. 2014, doi: https://doi.org/10.1093/brain/awu133.
- M. A. Kramer et al., “Human seizures self-terminate across spatial scales via a critical transition,” PNAS, vol. 109, no. 51, pp. 21116–21121, Dec. 2012, doi: https://doi.org/10.1073/pnas.1210047110.
- W.C. Chang et al., “Loss of neuronal network resilience precedes seizures and determines the ictogenic nature of interictal synaptic perturbations,” Nature Neuroscience, vol. 21, no. 12, pp. 1742–1752, Nov. 2018, doi: https://doi.org/10.1038/s41593-018-0278-y.
- S. Toprani and D. M. Durand, “Mechanisms of Neurostimulation for Epilepsy,” Epilepsy Currents, vol. 23, no. 5, pp. 298–302, Sep. 2023, doi: https://doi.org/10.1177/15357597231191887.
- W. W. Lytton, R. Orman, and M. Stewart, “Computer simulation of epilepsy: Implications for seizure spread and behavioral dysfunction,” Epilepsy & Behavior, vol. 7, no. 3, pp. 336–344, Nov. 2005, doi: https://doi.org/10.1016/j.yebeh.2005.06.011.
- C. U. Johannessen and S. I. Johannessen, “Valproate: Past, Present, and Future,” CNS Drug Reviews, vol. 9, no. 2, pp. 199–216, Jun. 2006, doi: https://doi.org/10.1111/j.1527-3458.2003.tb00249.x.