Spencer Fox Eccles School of Medicine
65 Alterations in Alzheimer’s Disease Phenotypic Features After Biodegradable Paclitaxel-Conjugate Treatment
Lana Hua; Donna Cross; Jiyuan (Jane) Yang; Shannou Li; Hasan Al Faruque; Raghad Aljassimi; Ashton Jensen; Jindřich Henry Kopeček; and Satoshi Minoshima
Faculty Mentor: Donna Cross (Radiology & Imaging Sciences, University of Utah)
Background
Alzheimer’s disease (AD) is characterized by key pathophysiological features including the accumulation of amyloid plaques and neurofibrillary tangles (NFTs). Amyloid plaques are specific to AD and consist of aggregates of β-amyloid peptides which are formed from amyloid precursor proteins (APP). NFTs are caused by misfolded tau which is a microtubule-associated protein that normally binds to microtubules and stabilizes them, however, in AD they become phosphorylated and dissociate from microtubules, forming the aggregates of which NFTs are composed.
This research proposes microtubule-stabilizing drugs, namely paclitaxel (PTX), as a potential therapeutic option for AD. PTX is typically used in cancer treatments, and it is unable to cross the blood-brain barrier (BBB), so it must be administered via the intranasal route. This places limits on the efficiency and efficacy of this treatment for AD. To address this, Dr. Cross’s team patented a PTX nano-conjugate that can cross the blood-brain barrier and uses a “long-circulating polymer-drug delivery technology” that makes this drug more ideal as a therapeutic option for AD and allows it to be administered intravenously in lower doses. PTX provides stability to microtubules by binding to them and preventing depolymerization, potentially rescuing cognitive deficits.
This research aims to evaluate whether the PTX-conjugate is a viable treatment for AD. Using biodistribution studies, the ability of the PTX-AP2 conjugate to cross the BBB was measured. Additionally, toxicity studies assessed whether the PTX-conjugate had adverse effects on cells and major organs. The changes in the hallmark pathophysiological features of AD, namely amyloid plaques and neurofibrillary tangles, after treatment with the novel PTX-conjugate were analyzed. Finally, the results of the Radial Water Maze were used to evaluate cognitive alternations in memory and spatial learning, following PTX-conjugate treatment.
Methods
Subjects
Wild type (WT) mice and “Triple transgenic Alzheimer’s (3xTg-AD) mice” with a mutation in APP. Subjects were randomly assigned to treatment groups (PTX-conjugate or vehicle).
Treatment
The novel PTX-conjugate was administered to 3xTg-AD mice ages 11-14 months to test the efficacy of the drug to change the progression of hallmark pathophysiological features such as basal neuroinflammatory responses and aggregated Tau as compared to previously published research with the generic PTX.
Assessments
- The HPMA copolymer was labeled with Cy5.5 and administered to mice. In-Vivo Imaging was taken for 72 hours following treatment to determine biodistribution.
- Radial Water Maze was conducted with 3 phases: learning and acquisition, short-term memory, and long-term memory. A two-factor ANOVA test and a Helmert a priori individual comparisons test were taken.
- Mice were euthanized, and their brains were collected for coronal slicing at 30 μm thickness. Slices were stained with glial fibrillary acidic protein (GFAP) or Iba-1. Sections were also stained with AT8 antibody to measure tau phosphorylation with a Dapi co-stain. Confocal microscopy images were obtained.
- IC50 values were measured using SHSY-5Y, a neuroblastoma cell line in mice, and bEND.3, endothelial cells, to determine cytotoxicity.
Results
Figure 1 – Synthetic scheme for the HPMA copolymer PTX-conjugate containing AP2
Angiopep-2 (AP2) is a 19-amino acid peptide sequence (TFFYG GSRGKRNNFKTEEY) functioning as a brain drug delivery agent that targets the low-density lipoprotein receptor related protein (LRP1). AP2 optimizes therapeutic efficiency by mediating transcytosis across the blood-brain barrier and using a “long-circulating polymer-drug delivery technology,” reducing adverse effects. It also avoids efflux-caused multidrug-resistance as it’s cell entry and subcellular fate mechanisms are different.
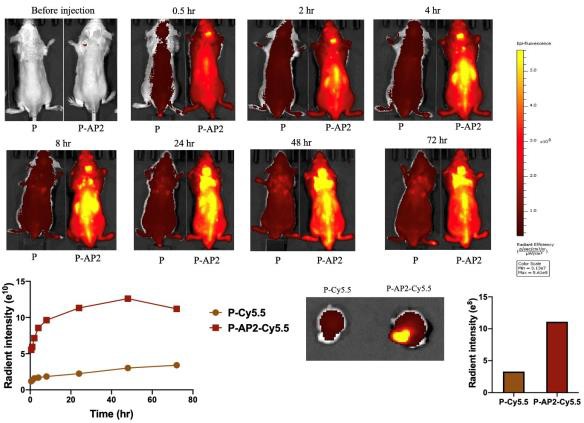
Figure 2 – Biodistribution studies demonstrate AP2 crossed the blood-brain barrier Biodistribution studies using a Cy5.5 fluorescence signal show that Angiopep-2 is distributed to the brain when conjugated to an HPMA copolymer after injection with PTX-conjugate. An In-Vivo Imaging System using Ex/Em 675/720 nm was used to measure the Cy5.5 signal 0.5, 2, 4, 8, 24, 48, and 72 hours post tail vein administration to the mice. The AP2-linked polymer showed high efficiency in crossing the BBB compared to the control.
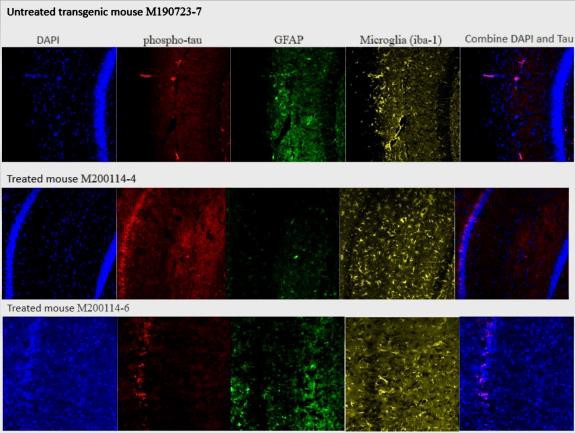
Figure 3 – PTX-conjugate alters phosphorylated tau and neuroinflammation in the hippocampus of aged 3xTg-AD mice from 11-14 months
Confocal microscopy images for 3xTg-AD mice treated with the PTX-conjugate or saline after the onset of pathology are shown. PTX-conjugate treatment showed reductions in microglial activation (iba-1) and reduced GFAP expression in astrocytes. A Dapi co-stain was used to co-localize the phospho-tau to the nuclei of the neurons.
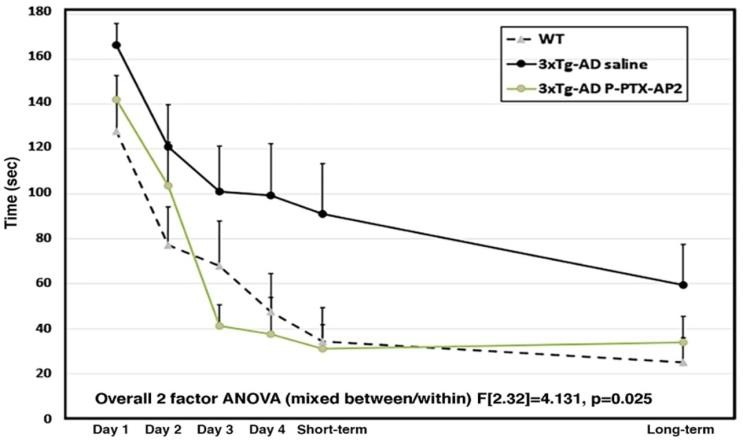
Figure 4 – P-PTX-AP2 Treatment Administered Via IV
The two-factor ANOVA indicated statistically significant improvements in treatment response with the PTX conjugate compared to the generic PTX in 3xTg-AD aged mice between groups (F[2,32]=4.131, p=0.025) and group by days interactions (F[2,32]=3.823, p=0.032). A Helmert a priori individual comparisons test indicated that the PTX-AP2-treated and WT mice performed significantly better than vehicle-treated 3xTg-AD mice (p0.007). PTX-AP2-treated mice did not differ significantly from WT (p0.921).
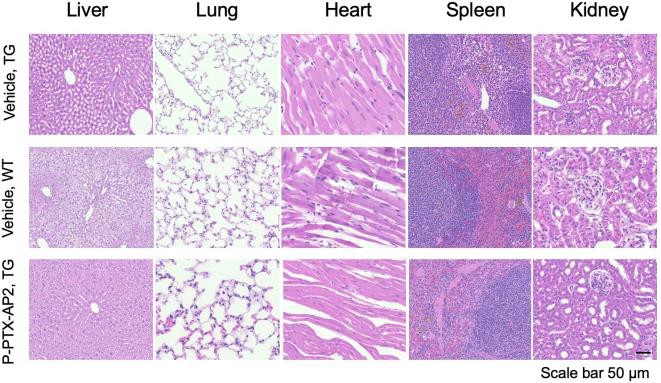
Figure 5 – PTX-conjugate effect on gross organ toxicity between treated and non-treated groups H&E stain showed no gross organ toxicity in the major organs of treated mice, indicating the absence of significant adverse effects on the liver, lungs, heart, spleen, and kidneys compared to the non- treated group.
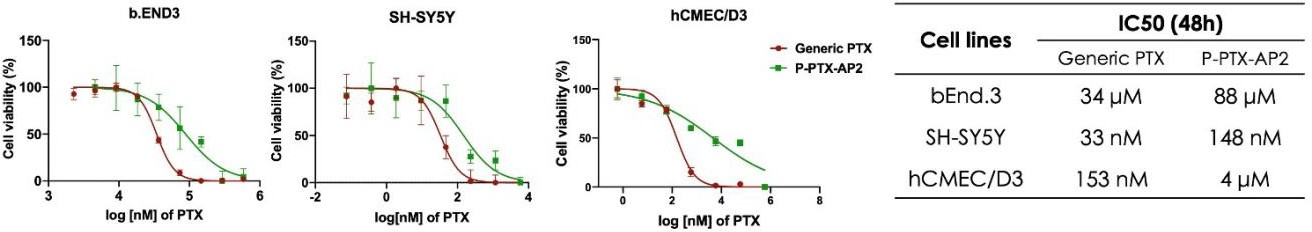
Figure 6 – Cytotoxicity of PTX-conjugate on bEND.3 and SH-SY5Y
bEND.3 and SH-SY5Y cell lines mimicked endothelial cells of the BBB and neurons, respectively. The IC50 values of the PTX-conjugate were higher for both cell lines compared to generic PTX.
Discussion
The novel PTX-conjugate contains Angiopep-2 (AP2), a peptide that functions to facilitate drug transport across the BBB (figure 1). Biodistribution studies demonstrated that AP2 reached the brain in the 3xTg-AD mice, optimizing the therapeutic efficacy of the drug and allowing for low-dose intravenous administration (figure 2).
Results showed alterations in phosphorylated tau and neuroinflammation in the histology of the 3xTg- AD coronal brain slices through the hippocampus (figure 3). These results were seen in aged mice treated with the PTX-conjugate after the onset of pathology from 11-14 months. Results of the Radial Water Maze indicate significant improvements in the cognition, specifically, spatial learning and memory, of PTX-conjugate treated mice compared to vehicle-treated mice (figure 4).
Finally, toxicity studies show no gross organ toxicity resulting from low-dose intravenous administration of the PTX-conjugate (figure 5). Additionally, cells tolerated higher concentrations of the PTX conjugate before cell death compared to those treated with generic PTX, indicating reduced cytotoxicity in the PTX conjugate (figure 6).
Conclusion
There are currently limited therapeutic options to treat AD, so there is a significant need for treatments that could positively impact the lives of those struggling with AD by improving cognition or reducing further cognitive decline.
Results of this study have positive implications in the pursuit of AD treatments. Biodistribution results indicate that AP2 facilitates drug transport across the BBB, allowing for intravenous administration.
Additionally, no gross organ toxicity was observed, and the cytotoxicity of the PTX-conjugate was less than that of generic PTX. Results from the Radial Water Maze showed that the PTX-conjugate was successful in rescuing cognitive deficits in aged 3xTg-AD mice.
Additional histology and immunostaining assays are to be completed and analyzed for alterations in key pathophysiological features of AD, including amyloid plaques and neurofibrillary tangles associated with neurodegeneration after treatment with the PTX conjugate. Further studies regarding biodistribution and pharmacokinetics using I-125 radiolabeled PTX-conjugate will also be conducted.
Bibliography
Cross, D. J., Huber, B. R., Silverman, M. A., Cline, M. M., Gill, T. B., Cross, C. G., … & Minoshima, S. (2021). Intranasal paclitaxel alters Alzheimer’s disease phenotypic features in 3xTg- AD mice. Journal of Alzheimer’s Disease, 83(1), 379-394.
Gouras, G. K., Olsson, T. T., & Hansson, O. (2015). β-amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease. Neurotherapeutics, 12(1), 3-11. https://doi.org/10.1007/s13311-014-0313-y
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., Cummings, J., & van der Flier, W. M. (2021). Alzheimer’s disease. The Lancet, 397(10284), 1577- 1590. https://doi.org/10.1016/S0140-6736(20)32205-4

