Faculty Mentor: Christopher Depner (Health and Kinesiology, University of Utah)
Abstract
Short sleep duration is linked to increased risk for metabolic disorders, including obesity, diabetes, and cardiovascular disease. Circadian misalignment, which can occur as a result of short sleep duration, exacerbates these risks by further disrupting glucose metabolism and impairing insulin sensitivity. Despite these well-documented effects, interventions promoting circadian alignment to mitigate these adverse health outcomes remain poorly researched. Thus, our goal was to assess the impact of a circadian-based intervention on insulin sensitivity and cardiometabolic health outcomes in overweight adults with habitual short sleep duration.
Methods: This part of the Timing of Circadian Synchronizers (TOCS) study investigated the effects of a circadian-based intervention on insulin sensitivity in overweight adults with habitual short sleep duration. The protocol consisted of a 9-week randomized controlled trial with control and intervention groups. Verbal instructions directed participants in the intervention group to optimize circadian timing using light interventions. The Matsuda Index (MI) assessed insulin sensitivity using oral glucose tolerance tests (OGTT) conducted at baseline and post-intervention. Wrist actigraphy data and self-reported sleep logs measured sleep parameters. Frequent saliva melatonin sampling during overnight visits determined circadian timing.
Findings: Preliminary results from this ongoing study suggest that the intervention did not significantly (p >0.05) impact circadian timing (DLMO and DLMOFF), insulin sensitivity (MI), sleep duration (TST), bedtime (BT), or wake time (WT). Additionally, baseline values of MI, TST, BT, and WT were strong predictors of their respective Week 8-9 values, suggesting that initial sleep and metabolic characteristics heavily influence later outcomes.
Interpretation: Preliminary analyses show the intervention did not significantly alter insulin sensitivity or circadian timing, though baseline measures were strong predictors of post-intervention values. Final analyses with the full sample size will help clarify the relationship between circadian timing and metabolic health outcomes. It may also be valuable to introduce an intervention that more specifically targets sleep timing or regularity as opposed to light exposure.
Introduction
Obesity and habitual short sleep duration are epidemic public health concerns that contribute to risk of metabolic disease including type 2 diabetes and cardiovascular disease, which are major causes of morbidity and mortality (Cappuccio et al., 2008). As of 2021, “537 million individuals globally were affected with diabetes” (Hossain et al., 2024). This number is “predicted to increase to 784 million by 2045” (Singer et al., 2022). The prevalence of diabetes is dangerously high and is projected to rise rapidly over the next few years based on current trajectories. Given these alarming statistics, there is a critical need for health-based interventions that mitigate this epidemic of cardiometabolic disease in modern society.
In parallel with the increased incidence of diabetes, there has been a concomitant decrease in average nightly sleep duration (Cappuccio et al., 2008). Additionally, sleeping less than the recommended 7 hours per night is an independent risk factor for Type 2 diabetes mellitus and Metabolic Syndrome (Eckel et al., 2015; Depner et al., 2014). There is a notable correlation between lack of sleep and reduced insulin sensitivity which is subsequently associated with type 2 diabetes (Depner et al., 2014). Data from our lab show even short- term sleep restriction of 5 days in otherwise healthy adults significantly impairs insulin sensitivity (Eckel et al., 2015). Alarmingly, the impaired insulin sensitivity in this study due to sleep loss was severe enough that some participants met criteria for pre-diabetes, demonstrating the adverse consequences of short sleep are clinically meaningful. In aggregate, these data highlight and stress the importance of obtaining optimal sleep to promote overall metabolic health.
Addressing and managing sleep habits could have a significant impact on insulin sensitivity and related risk of cardiometabolic disease. However, to date, there are no established free- living interventions to mitigate the adverse cardiometabolic risk of short sleep duration. One potential mechanism to address is circadian misalignment, which a leading expert defines as “inappropriately timed sleep and wakefulness” (Baron, 2014). Prior data from our lab shows the magnitude of circadian misalignment during sleep loss is associated with the magnitude of impaired insulin sensitivity, suggesting circadian misalignment is a potential mechanism underlying the link between short sleep and risk of diabetes (Eckel et al., 2015; Duraccio et al., 2024). To address this knowledge gap, we will investigate the impact of a circadian intervention on insulin sensitivity and sleep duration in a free-living setting. A real-world setting will further bridge the gap in understanding the impact of short sleep on insulin sensitivity.
Methods
Participants
Recruitment targeted adults aged 18–45 years with a BMI of 25.0-34.9 kg/m² and habitual sleep duration of <6.5 hours per night. Exclusion criteria included diagnosed sleep disorders, major psychiatric illnesses, significant organ dysfunction (e.g., cardiovascular disease or kidney disease), or use of medications affecting sleep or metabolism. To date, 15 out of a planned 28 adults (7 control, 8 intervention) aged 28.7 ± 7.9 years, BMI 29.2 ± 2.1 kg/m2 [mean ± SD] have completed data collection.
Study Design
We conducted a 9-week randomized controlled trial, during which all participants underwent a 1-week baseline testing followed by an 8-week intervention. Participants were randomized (1:1) to either a circadian intervention group or a healthy lifestyle control group, stratified by sex. The circadian intervention involved wearing blue light-blocking glasses starting four hours before bedtime and attaining light exposure for the first hour after waking via a light box or blue light emitting glasses. After baseline and intervention segments, participants completed an OGTT followed by overnight melatonin sampling (Figure 1). Wrist actigraphy devices measured sleep parameters throughout the study, and self-reported sleep logs supplemented actigraphy data.
Data Processing
RStudio analyzed wrist actigraphy data using the GGIR package (version 3.0-6). Standard algorithms within GGIR extracted sleep parameters, including TST, BT, and WT. GGIR automatically detected and excluded non-wear time. Manual data quality checks ensured completeness and accuracy, and excluded those participants whose data was insufficient.
Statistical Analysis
Analysis of Covariance (ANCOVA) evaluated the effect of the intervention while controlling for baseline values and potential confounding variables. Paired-sample t-tests assessed within-group changes for statistically significant differences in outcome measures from baseline to post-intervention. Two participants in the control group were missing wrist actigraphy data due to device malfunctioning during the intervention segment, which required their exclusion from sleep analysis. The study excludes one subject who was an outlier in DLMO. Presented data are mean ± SEM.
Results
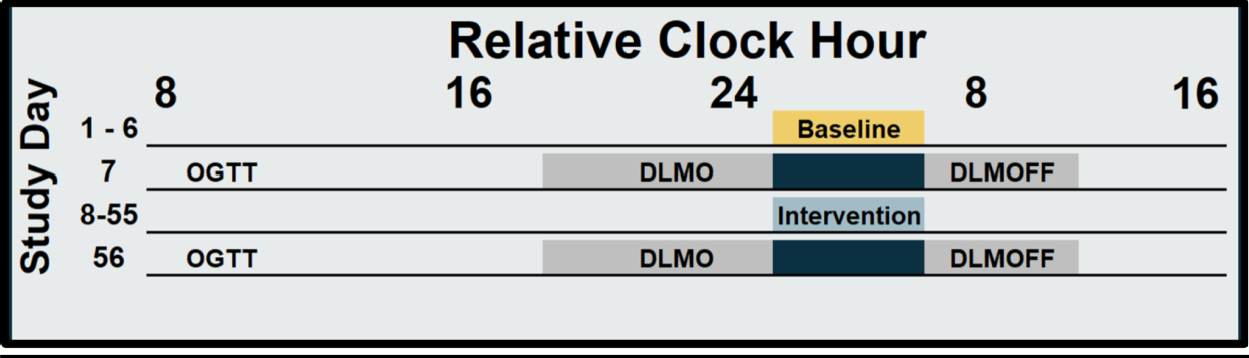
Figure 1. Protocol. Solid bars indicate sleep opportunities. Yellow bar indicates baseline habitual short sleep (Days 1-6),light blue bar indicates the 8-week intervention segment (Days 8-55) and dark blue bars (Days 7 & 56) indicate the overnight in laboratory testing including OGTT, DLMO and DLMOff. Grey bars indicate dim light conditions during DLMO and DLMOff.
Dim Light Melatonin Onset and Offset (DLMO and DLMOFF)
At baseline, the experimental group had a DLMO of 21.46 ± 0.67 hrs, and after the intervention DLMO was 20.91 ± 0.53 hrs. This data showed a statistical trend with p = 0.062. The control group at baseline had a DLMO of 22.58 ± 0.79 hrs, which was not significantly different from the control group post-intervention at 21.54 ± 0.31 hrs.
The experimental group at baseline had a DLMOFF of 9.46 ± 0.49 hrs, which was not significantly different following the intervention at 9.75 ± 0.67 hrs. At baseline, the DLMOFF of the control group was 7.75 ± 0.53 hrs, which was not significantly different from the control post-intervention at 7.79 ± 0.48 hrs.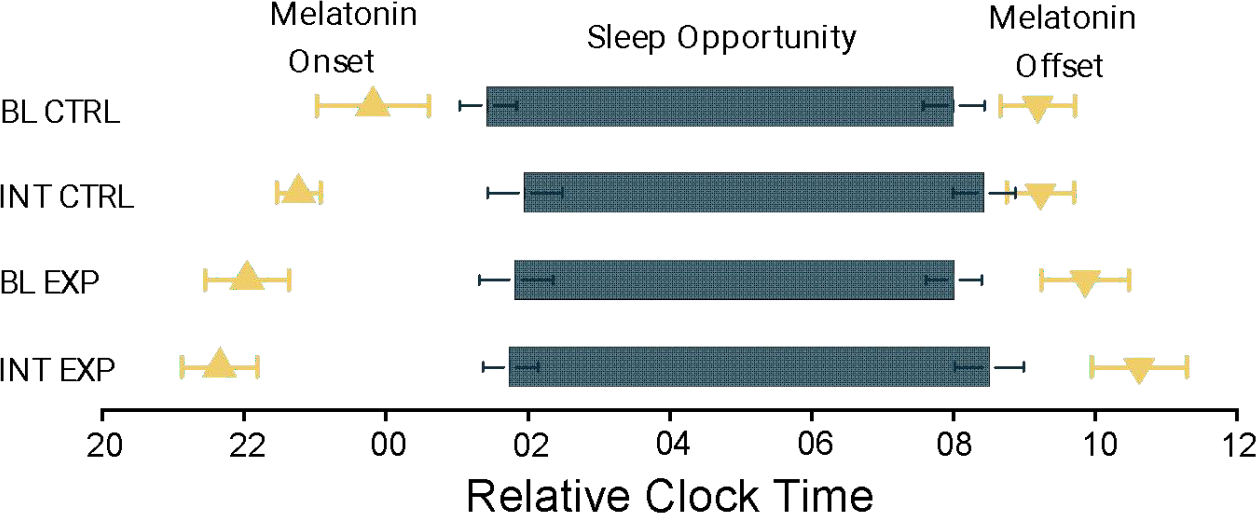
Figure 2. Dim Light Melatonin Onset (DLMO) and Dim Light Melatonin Offset (DMLOFF) with Bed and Wake Time during Baseline and Intervention.
Total Sleep Time (TST)
At baseline, the experimental group had a TST of 5.56 ± 0.15 hours. TST was similar following the intervention, at 5.86 ± 0.23 hours. At baseline, the control group had a TST of
5.89 ± 0.47 hours. TST was similar at the end of the experiment at 5.73 ± 0.32 hours. There was a significant moderate positive correlation between TST in the experimental group at baseline and post-intervention (r = 0.61, p < 0.05).
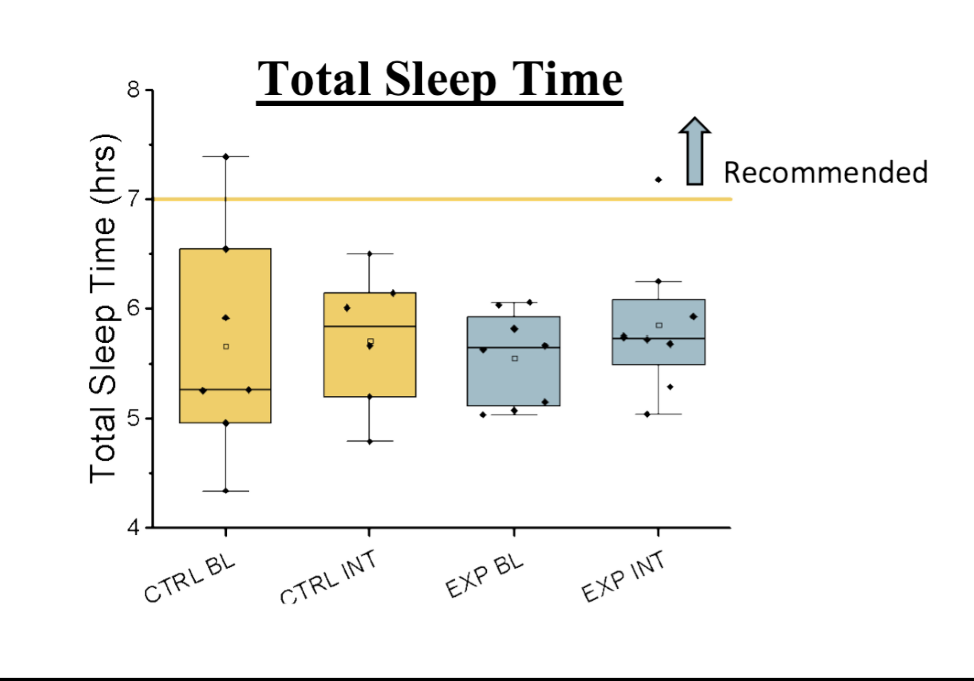
Figure 3. TST during Baseline, and Intervention Weeks 8-9. TST was not significantly different between groups.
Wake Time (WT)
At baseline in the experimental group, WT was 7.30 ± 0.34 hours which was not significantly different (p > 0.05) following the intervention 7.67 ± 0.43 hours. At baseline in the control group, WT was 6.72 ± 0.48 hours which was not significantly different (p > 0.05) following the intervention 7.15 ± 0.44 hours. There was a significant strong positive correlation between WT in the experimental group at baseline and post-intervention. (r = 0.72, p < 0.05)
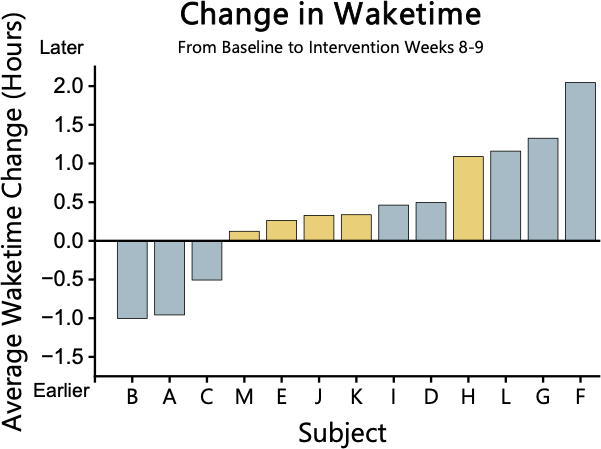
Figure 4. Change in WT from baseline to Intervention weeks 8-9. WT is not significantly different between groups.
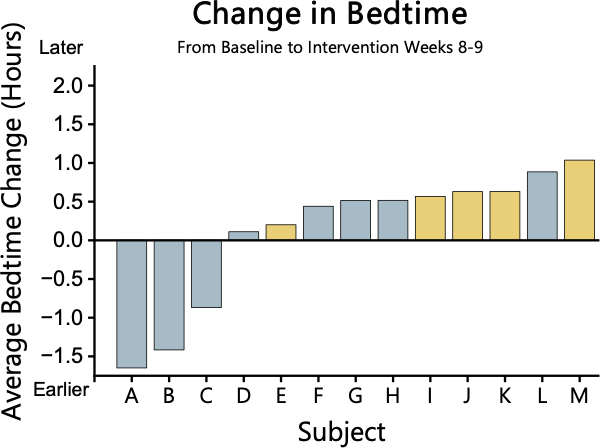
Bed Time (BT)
Figure 5. Change in BT from baseline to Intervention weeks 8-9. BT is not significantly different between groups.
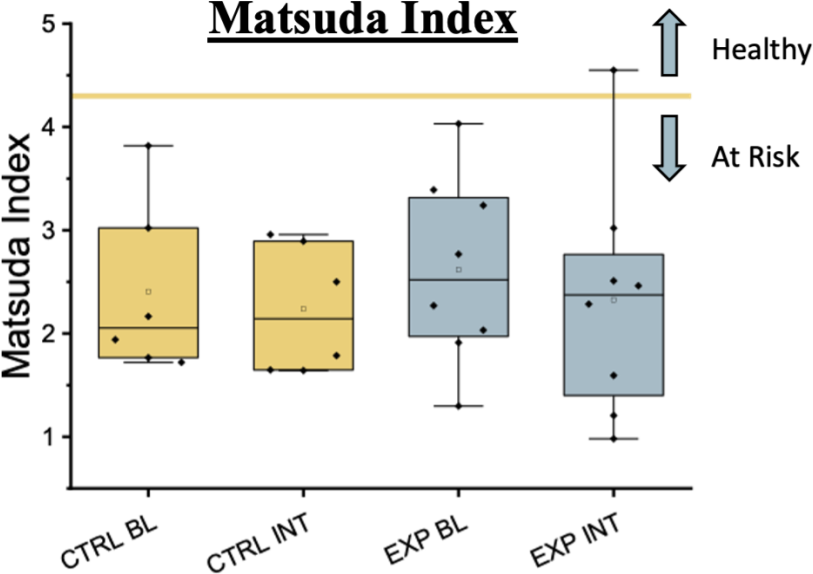
Matsuda Index (MI)
The intervention did not significantly impact the MI (p > 0.05). The experimental group had a MI of 2.61 ± 0.32 at baseline and 2.33 ± 0.40 post-intervention. In the control group, the MI baseline was 3.03 ± 0.69 which was not significantly different at the end of the experiment at 2.79 ± 0.59. There was a significant strong positive correlation between MI in the experimental group at baseline and post-intervention. (r = 0.74, p < 0.05)
Figure 6. MI during Baseline, and after the Intervention. MI was not significantly different between groups.
Conclusion
Currently, 15 out of 28 participants have completed the study; however, our preliminary findings indicate that there are no significant differences between groups following the intervention in DLMO, DLMOFF, TST, WT, BT, or MI. Current analysis shows a trend for an earlier DLMO in the intervention group, consistent with our hypothesis that the light-based intervention would shift circadian timing earlier. If this trend continues with the full sample size we will have adequate statistical power to detect such a shift in DLMO. Despite this potential positive shift in DLMO in the experimental group,. there were no significant differences in the Matsuda Index, suggesting no benefit on diabetes risk. Moreover all our participants’ baseline Matsuda Index was at a level below 4.3 which is considered insulin resistant (Takahara et al., 2013), and thus all our participants were at elevated risk of diabetes at baseline. It is possible that other lifestyle factors such as diet, physical activity, and external stressors may have influenced insulin sensitivity more heavily than the implemented light-based intervention. A strength of this study is that although the light-based interventions did not produce as large of an impact on circadian timing as hypothesized, the study occurred in a real-world setting where natural sunlight and other factors could still influence circadian timing. Thus, our findings show feasibility of participants to complete the light-based intervention but did not show strong efficacy for the intervention to impact circadian timing or risk of diabetes. This is valuable knowledge because it demonstrates that translating findings from controlled laboratory studies into the free-living environment, regarding circadian based interventions, is complex and not a one-to-one translation. This knowledge will directly inform the design of new and more efficacious interventions designed to mitigate the risk of diabetes linked to short sleep duration. Ultimately, these data suggest that an intervention targeting sleep timing instead of light exposure could be more influential in modifying circadian rhythms and improving cardiometabolic outcomes. Data collection is still ongoing, and a full data set is required to determine if these results will remain constant.
Acknowledgements
I would like to thank Audrey Stegman, Dr. Christopher Depner and the research team for their guidance, and support throughout this process. The parent study “Timing of Circadian Synchronizers,” from which the data for the current project were collected, was funded by the National Institutes of Health through grants NIH- UL1TR002538; NIH-K01HL145099; NIH-R01HL166733; NIH-T32DK110966; as well by University of Utah Seed Grant-10060570, Ben B. and Iris M. Margolis Foundation. The current project was supported by UROP from the Office of Undergraduate Research at the University of Utah.
Bibliography
Baron, K. G., & Reid, K. J. (2014). Circadian misalignment and health. https://pubmed.ncbi.nlm.nih.gov/24892891/
Cappuccio, F. P., Taggart, F. M., Kandala, N.-B., Currie, A., Peile, E., Stranges, S., & Miller,
M. A. (2008). Meta-analysis of short sleep duration and obesity in children and adults. Sleep, 31(5), 619–626. https://doi.org/10.1093/sleep/31.5.619
Depner, C. M., Stothard, E. R., & Wright, K. P. (2014). Metabolic consequences of sleep and circadian disorders. Current Diabetes Reports, 14(7). https://doi.org/10.1007/s11892-014- 0507-z
Duraccio, K. M., Kamhout, S., Baron, K. G., Reutrakul, S., & Depner, C. M. (2024). Sleep extension and cardiometabolic health: What it is, possible mechanisms and real‐world applications. The Journal of Physiology. https://doi.org/10.1113/jp284911
Eckel, R. H., Depner, C. M., Perreault, L., Markwald, R. R., Smith, M. R., McHill, A. W., Higgins, J., Melanson, E. L., & Wright, K. P. (2015). Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Current Biology, 25(22), 3004–3010. https://doi.org/10.1016/j.cub.2015.10.011
Hossain, Md. J., Al‐Mamun, Md., & Islam, Md. R. (2024). Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Science Reports, 7(3). https://doi.org/10.1002/hsr2.2004
Singer, M. E., Dorrance, K. A., Oxenreiter, M. M., Yan, K. R., & Close, K. L. (2022). The type
2 diabetes ‘modern preventable pandemic’ and replicable lessons from the covid-19 crisis.
Preventive Medicine Reports, 25, 101636. https://doi.org/10.1016/j.pmedr.2021.101636
Takahara, M., Katakami, N., Kaneto, H., Noguchi, M., & Shimomura, I. (2013). Distribution of the matsuda index in Japanese healthy subjects. Journal of diabetes investigation. https://pmc.ncbi.nlm.nih.gov/articles/PMC4020231/
About the authors
name:
Christopher Depner
institution:
University of Utah