College of Health
37 Impacts of High-Fat Diet Feeding on the Metabolic Health in Ovariectomized Mice
Ceyda Ural
Abstract
Newly postmenopausal women are faced with an increased risk of obesity– including weight gain and body fat accumulation– and cardiometabolic dysfunction. Obesity affects almost half of the population and is a condition of metabolic syndrome; with an elevated risk of metabolic, cardiovascular, and neurological disorders. Although the mechanisms are unknown, this metabolic behavior may be related to estrogen levels, as estrogen can affect the accumulation and distribution of adipose tissue and is described to have a protective effect on the cardiovascular system.
To explore estrogen’s involvement in metabolic processes, we will be replicating a well-established model of diet-induced obesity of C57BL/6J male mice being fed on a high fat diet. Using C57BL/6J female mice, we will observe the effects of diet as well as their hormonal and reproductive states. This postmenopausal model will be replicated by surgical ovariectomies and will be compared to mice that undergo a sham surgery with differing diet interventions. The diets that will be used as intervention will be normal chow, consisting of 20% fat, or 60% high-fat diet to investigate diet composition’s effect on cardiometabolic health in postmenopausal mice. To analyze the effects on their metabolic health, the data will consist of food consumption, body weight, body composition, and blood serum analysis.
After 10 weeks of diet intervention, the OVX mice on HFD gained significantly more body weight and fat mass than the Sham mice on the HFD. These findings suggest there is a relationship between diet composition, fat accrual, and estrogen levels in this B6 model of diet-induced obesity.
Introduction
Obesity continues to be an epidemic, especially in the United States. Excess weight gain has an overarching effect on the health and well-being of an individual. In particular, as the rates of obesity increase, so do the rates of many metabolic diseases. Metabolic syndrome (MetS) is defined by conditions that frequently occur together -including increased waist circumference, hypertension, dyslipidemia, and insulin resistance- and causes an increased risk of diabetes and cardiovascular diseases (1,3,4,6). It’s predicted that type 2 diabetes mellitus will affect over 400 million people by the year 2030; those with a type 2 diabetes mellitus diagnosis are more likely to be obese than nondiabetics (1,2). Furthermore, around 65%-75% of the risk of developing hypertension can be directly tied back to obesity (1). An indicator of metabolic dysfunction can be observed via blood sample analysis for reduction of high-density lipoprotein cholesterol or increased triglyceride content (4).
Menopause is when a woman’s ovaries stop producing hormones and their menstruation ceases. Prior to menopause, the ovaries produce estrogen hormones that are essential for fertility but also can affect metabolic health. In particular, estrogen has been linked to fat accumulation and as a protective agent against cardiovascular diseases (5,6). When examining the interaction between reproductive states and metabolic dysfunction, the mechanisms are still unclear and there are many factors to consider. Weight gain is a common occurrence in postmenopausal women, specifically an increase of central fat mass (3,5). This body fat accumulation, resulting in an increased waist circumference which is associated with MetS, may be due to the estrogen deficient state (5). Menopause is also associated with an increased cardiovascular risk and this may be due to reduced lipid usage. Estrogen is produced in the ovaries with circulating cholesterol (5). Through menopause, this process is halted and causes an increase in total cholesterol in the bloodstream leading to an increased risk in cardiometabolic dysfunction (6,7). There is evidence that total cholesterol levels tend to rise in newly post-menopausal women and triglyceride levels only vary based on body weight (6,7). Although it is still disputed, the relationship between sex hormones and certain lipids and fatty acids may explain the physiological changes women can experience as they go through menopause.
Estrogen replacement therapy is a controversial topic because some studies show its benefits and others show detrimental side effects. As of now, hormone replacement therapy is optional for women. Studies indicate estrogen replacement therapy reduces the risk of MetS and diagnosis of cardiovascular disease, osteoporosis, and eases symptoms of vasomotor instability (3,8) However, there are also some negative side effects to estrogen replacement intervention. In a clinical study where participants took conjugated estrogen orally there was a 20% increased risk of endometrial hyperplasia after one year (8,9). There are various factors that may be affecting these results like how long supplemental estrogen is used and if the dosing is paired with other hormones. For example, that same study tested the use of medroxyprogesterone along with the conjugated estrogen and found it can mitigate the risk of endometrial hyperplasia (8,9). As such, there are many facets of supplemental hormone therapies that need to be understood before calling it a solution.
The vast majority of preclinical research on MetS has used male mice. In most cases (including this project), researchers use the C57Bl/6J strain of mice, because of their genetic homogeneity and their high susceptibility to diet-induced obesity and associated metabolic diseases. However, if males are highly susceptible, females are not. In fact, there is a strong sexual dimorphism in their response to diet-induced obesity so that females on HFD gain much less weight than the males (5,11). Although this research has been key in discovering mechanisms and developing new therapies, this causes obstacles when applying these findings to females. Ultimately, pre-clinical research utilizes scientific tools like mice due to their genetic similarities and aims to translate their findings to humans. When there are innate differences between males and females across all domains of life, focusing on just the males causes limitations and a vast gap in our knowledge. For example, the risk of females developing metabolic diseases can vary but after diagnosis, they’re prone to progressing faster and to more severe forms of such diseases (12). In this research, we chose to characterize diet-induced obesity and associated metabolic disturbances in ovariectomized females to increase our understanding of MetS in the other half of the population. This focus aims to develop an overall understanding of the entire population’s metabolic behaviors. This is essential since the drawback of producing therapeutics based solely on one-sided research could potentially result in treating only half of the population.
Methods
Mice
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Utah. For this study, 5-week-old C57BL/6J female mice purchased from Jackson Laboratories (Bar Harbor, ME) were used and acclimated for 3 weeks before the OXV or Sham surgery; mice were fed a NC diet for the acclimatization period and for the 2 week healing period. All mice in the study were housed under 12:12-hour dark-light cycle and given ad libitum access to food and water
Ovariectomy and sham surgery
Mice were shaved and cleaned with iodine and alcohol wipes. After anesthetizing the mouse, a small incision (approximately 2 cm) was made along the lower back of the mouse. Following this incision, two small incisions in the peritoneum (approximately 3-5 mm) were made to grasp the ovarian fat pad. The ovary was removed without damaging the uterus and the ovarian fat pad was pushed back into place. The incisions in the peritoneum were sutured closed with a continuous stitch using absorbable sutures. The incision in the skin was closed with wound clips spaced 2mm apart. The sham group underwent a similar surgery without the removal of the ovaries. Sham mice represent controls with premenopausal levels of estrogen. Post-surgery, the mice were monitored two times a day for a two-week recovery period. Ointment was reapplied on the incision site as needed. In addition to observing the healing of the skin, extreme changes in body weight were also monitored.
Diets
Two diets were used in this experiment: Normal chow (Research Diets D10012G, 20%kcal from fat) and High Fat Diet (Research Diets D12492, 60% kcal from fat). All cohorts received unrestricted access to food throughout the day.
Body weight and food consumption
At the beginning of intervention, changes in body weight were measured at least two times a week. As weight gain stabilized, the body weight of each mouse was recorded weekly. Additionally, the food consumption was recorded weekly by documenting the amount of food that was given and how much food was left after a week.
Body composition
The body composition of the mice was recorded using a Bruker’s minispec mq20, a benchtop TD-NMR. This equipment records radio frequency signals from the hydrogen in soft tissue and distributes the different NMR signals with different body tissues for body composition estimates (13). These measurements were done monthly with each mouse being weighed and inserted into the machine to analyze changes to their fat and lean content.
Blood analysis
At 20 weeks old, blood samples from the OVX mice were collected to do serum biochemistry analysis. These samples were collected by submandibular blood collection; puncturing the submandibular vein halfway between the ear and the mandible. Blood was collected with collection tubes and pressure with gauze was used to stop the bleeding. The blood serum analysis consisted of doing a lipid panel to test for triglyceride, cholesterol, and nonesterified fatty acid concentrations (NEFA). Additional testing of insulin levels allowed for an understanding of how the mice regulate their body’s energy supply. For the analysis, use a standard curve for comparison to ultimately calculate concentrations from the recorded serum absorption spectra.
Statistics
GraphPad Prism 6 was used to calculate the statistical analysis reported in this thesis. We used a 2-way repeated measure ANOVA to estimate p-values and significance was defined as 𝑝 < 0. 05, where p is a measure of how likely these results occurred by chance.
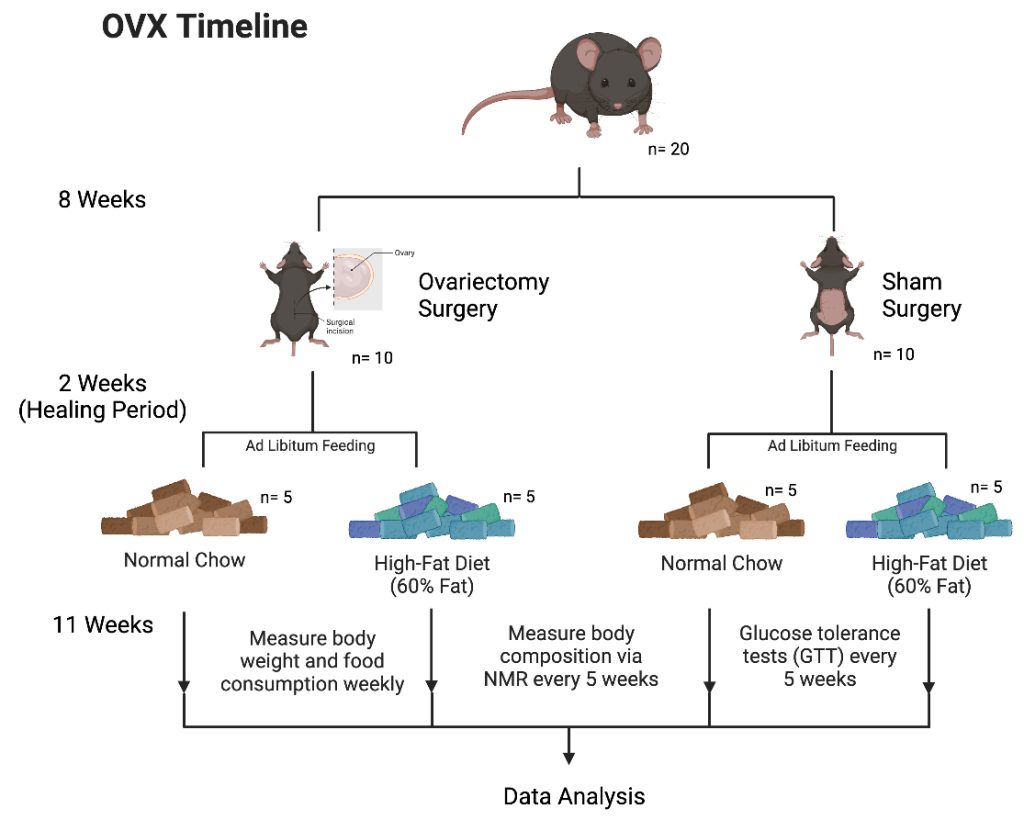
Figure 1. Experimental Design.
Results
This experiment focused on the impact of female mice with and without their ovaries on metabolism and body weight regulation. Using female C57BL/6J mice at 7 weeks old, the groups were randomly selected. One group undergoes the ovariectomy surgery (OVX) and the other undergoes a sham surgery (Sham). After a two week recovery period, the two groups were divided again into a total of 4 groups: OVX mice on normal chow (NC+OVX), OVX mice on High Fat Diet (HFD+OVX), Sham mice on NC, and Sham mice on HFD. In order to analyze the effects of the OVX surgery, changes in body weight, body composition, and food consumption were recorded. Additionally, blood samples were collected to do serum biochemistry.
Change in Body Weight
As observed in Figure 2, the weight gain progressively became more drastic for the HFD+OVX from its Sham HFD counterparts. The HFD+OVX group had an average body weight of 39.102±3.387 grams in comparison to the HFD Sham group, which had an average of 31.312±2.29 grams. When calculating the p value with a 2-way repeated measure ANOVA test, it resulted in 𝑝 < 0. 0001. For the other diet tested, there was not a significant difference between the NC+OVX mice from the Sham NC group. However, the HFD group gained significantly more body weight than the NC group and supports the previous research done on diet-induced obesity in C57BL/6J male mice. The NC Sham mice had an average body weight of 22.995±0.858 grams at the end of the study. There is approximately a 9 gram difference between the Sham groups, supporting the diet-induced obesity model translation in C57BL/6J female mice. The calculated p value was 𝑝 < 0. 0001, allowing for the assumptions that the difference in weight gain is correlated to the difference in diet composition.
Change in Body Fat Mass
Similarly, in Figure 3, the HFD+OVX group gains more fat mass over time when comparing the Sham HFD group. At the end of the experiment, the HFD+OVX group had an average fat content of 21.9±3.069 grams whereas the HFD Sham group had an average of 13.8±2.452 grams. The interaction between time and reproductive state for the HFD groups resulted in a p value ≤ 0. 031. Additionally, the Sham mice on HFD accumulated more fat mass than those on the NC diet. The Sham group on NC ended the study with an average 4.225±0.506 grams of body fat and the interaction between time and diet intervention has a significance of 𝑝 ≤ 0. 0005 to support the diet-induced obesity model. These p values help us deduce these results did not occur by coincidence and there is an interaction between fat mass, estrogen deficiency, and diet composition.
Food Consumption
Weighing the food given at the beginning of the week and subtracting the leftovers results in the food consumption for the week. This, with the logistics of number of animals in the cage and diet caloric density, allows calculations of food consumption in kilocalories per mouse.
This can be seen in Figure 4 and there is not a significant difference in intake between the HFD+OVX and Sham HFD groups. Towards the end of the study, both groups were consuming an average of 11-12 kilocalories per mouse per day. It is curious there was an increase in weight gain for the HFD+OVX when both groups were consuming a comparable amount of kilocalories.
Blood Serum Analysis
The HFD and NC had no significant difference in the triglyceride concentrations for either OVX group. There was a significant increase in cholesterol levels for the HFD+OVX group when compared to the NC+OVX group. These results allow a comparison between diets’ effects but not the effects of estrogen deficiency on these biomarkers.
Discussion
This study aimed to further the understanding of the protective role of female hormones on metabolic health by characterizing the effects of a surgical ovariectomy (OVX) on body weight, fat mass accrual, and other biomarkers of MetS. The removal of an important and distinct reproductive organ was capable of significantly impacting the metabolic health of these mice by increasing their weight gain and fat mass. Although further research is necessary to understand the mechanisms, these results suggest that the ovaries, or more specifically the gonadal hormones, significantly impact the risk of MetS and physiological responses to diet compositions.
The HFD+OVX group had a significant gain in weight and fat mass when compared to the HFD Sham group. The mice in both groups were fed a 60 kcal% HFD and had a similar daily food consumption. Due to the diet being the same between these two groups, the results suggest the effects of hypoestrogenism, or estrogen deficiency, is a factor in these physiological changes. The NC+OVX and Sham NC did not have as significant of a change in either their body weights or their fat mass accumulation. With the lower fat diet groups not having the same interaction as the high fat diet groups, this suggests the diet composition has a role in the relationship between hypoestrogenism and MetS.
With prior research and understanding of diet composition’s effect on metabolic health, the Sham HFD group had a significant increase in weight and fat mass compared to the Sham NC. This was expected, as it is a well-described model in the male counterparts of this species, and is directly related to the diet composition. Another factor we were able to explore was the effects of each diet on triglyceride and cholesterol levels in the OVX mice. Although the triglyceride levels were the same for both the NC+OVX and the HFD+OVX, the cholesterol levels were impacted. The HFD+OVX group had a significantly higher cholesterol concentration in the blood samples we collected at the end of the study than the NC+OVX. This finding further supports the effects of a 60%HFD and its ability to cause diet-induced obesity in these mice and conditions of MetS.
In the human context, this study gives perspective of how limited metabolic research is when solely using male models to develop therapies. Postmenopausal women face a myriad of risks and symptoms once their body naturally begins to cease menstruation. Although there may be potential therapies on the market, these treatments have a risk of treating the wrong things. There aren’t any equivalents of menopause and a post- menopausal state in males. When there is research and conclusions made on male metabolic health, the lack of consideration of sexual dimorphism can put females seeking answers at a disadvantage. Overall, there is a very obvious lack of representation in scientific research. One study found an apparent pro-male publication bias- where research on men was twice as likely to be published- leading to a paucity of information about the other half of the population (10). Our study attempts to even out the playing field with a focus on females and female processes. The results underline the impact of functioning ovaries on the health of a female mouse. The significant differences found between the HFD+OVX versus HFD Sham groups but not in the NC groups drives the importance of diet composition in the metabolic response in estrogen deficient females. The importance of diet on one’s health is a well known fact but in this data a fattier diet created a greater rift between the OVX and Sham groups. The mice that continuously stuck to a low fat diet did not have as significant of a difference in their metabolic health between the OVX and Sham groups. Ultimately, the results suggest there is a relationship between diet composition, fat mass accrual, and estrogen levels in the B6 model of diet-induced obesity. Additionally, the surgical removal of the ovaries as a model to study female-specific changes in metabolic status as a function of reproductive state is efficient.
It is worth noting there were many events of human error when collecting the data for this experiment. With the food consumption, we were unable to compare the kilocalories per mouse per day for all the groups because of miscommunications and this prevented accurate data from being collected for the NC groups. Additionally, at the end of the study, we collected the blood samples of the OVX groups but unfortunately did not for the Sham groups. Without the control groups, this stunted our analysis and only allowed conclusions to be drawn upon the effects of diet on triglyceride, cholesterol, NEFA concentrations between the OVX groups. It took away from our ability to analyze the effects of estrogen deficiency on the lipid concentrations in the blood. These are factors to consider when using this model in a repeated cohort to have a better refined experiment and a more successful results section.
With these results, there is a lot of room to expand and explore. Future directions would be testing other diet compositions to continue assessing the relationship diet composition and estrogen levels have in impacting metabolic health. Furthermore, other biomarkers like high or low-density lipoprotein cholesterol and estrogen levels should be tested throughout the study to understand how they may change throughout intervention.
With all the controversy and uncertainty surrounding estrogen-replacement therapy, testing a group that receives supplemental estrogen and comparing the data could help answer more of these unknowns. When hormone replacement therapy causes increased risks of cancers and other disorders, it contradicts our current understanding of estrogen’s anti-inflammatory properties. As such, including groups that receive supplemental estrogen can be relevant and informative for comparison to further our understanding of the mechanisms that are disturbed by estrogen levels. There are many facets of metabolic health that can be affected by hypoestrogenism; it is likely a combination of several mechanisms that cause changes to adiposity distribution and weight and an ultimate increase in risk of metabolic syndrome.
Acknowledgements
I am grateful to Amandine Chaix for her mentorship throughout this entire process. I would like to thank Molly Gallop for helping see this vision through. Also, I would like to thank Sofia Whitefields and Elijah Matsuzaki for always answering an abundance of questions. Lastly, I want to thank the LEAP program and UROP for creating these opportunities and connecting me to the Chaix lab.
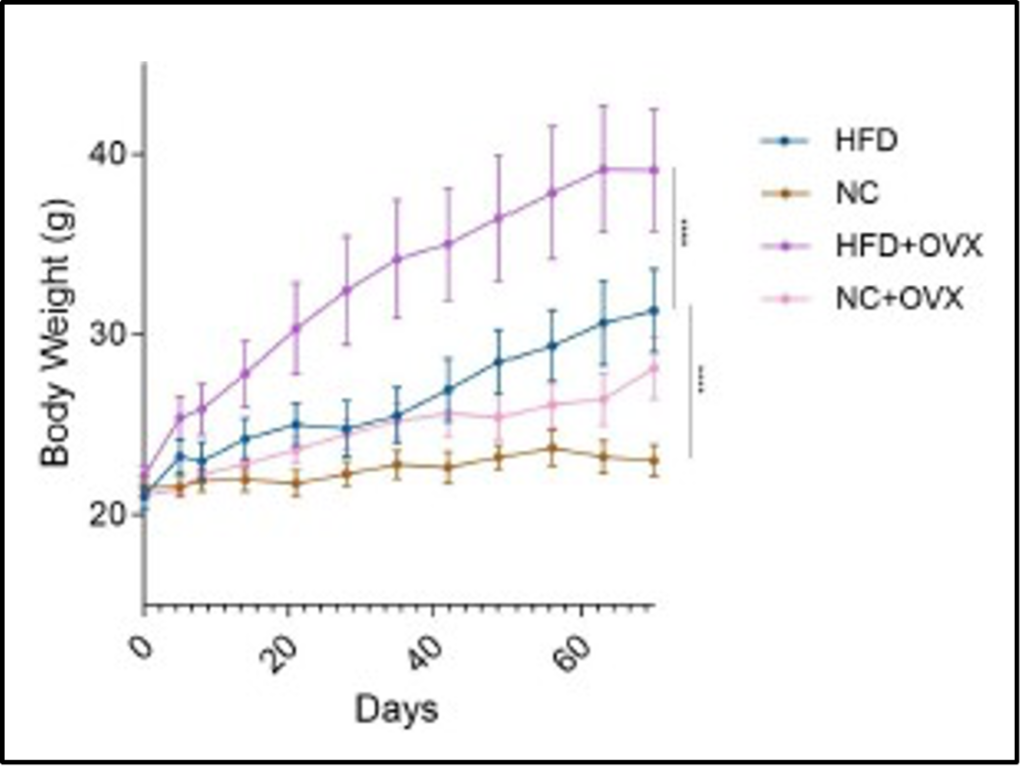
Figure 2. The Change in Body Weight Over Time.
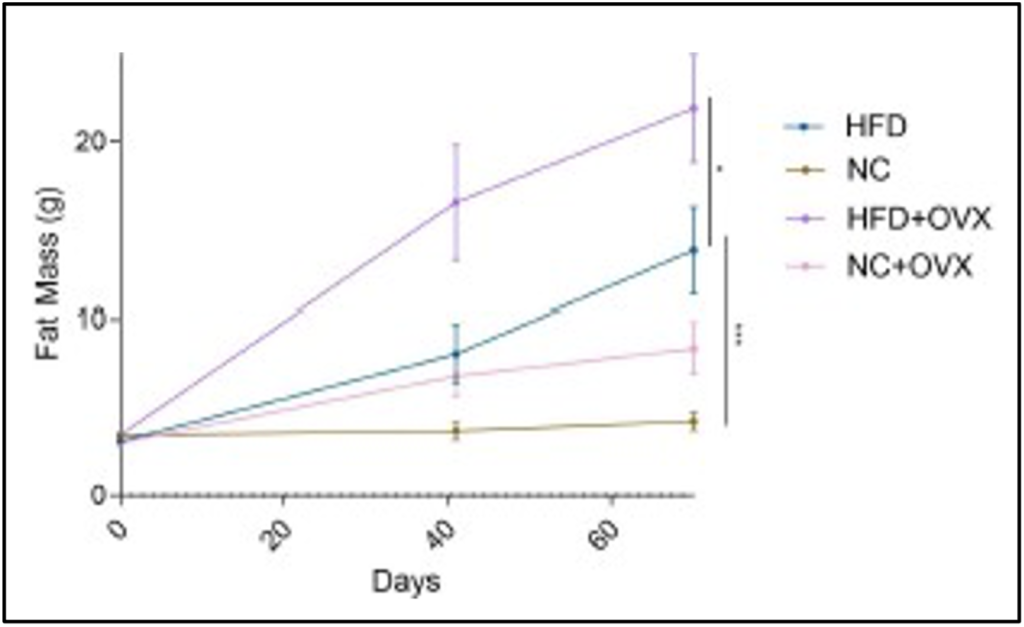
Figure 3. The Change in Fat Mass Over Time.
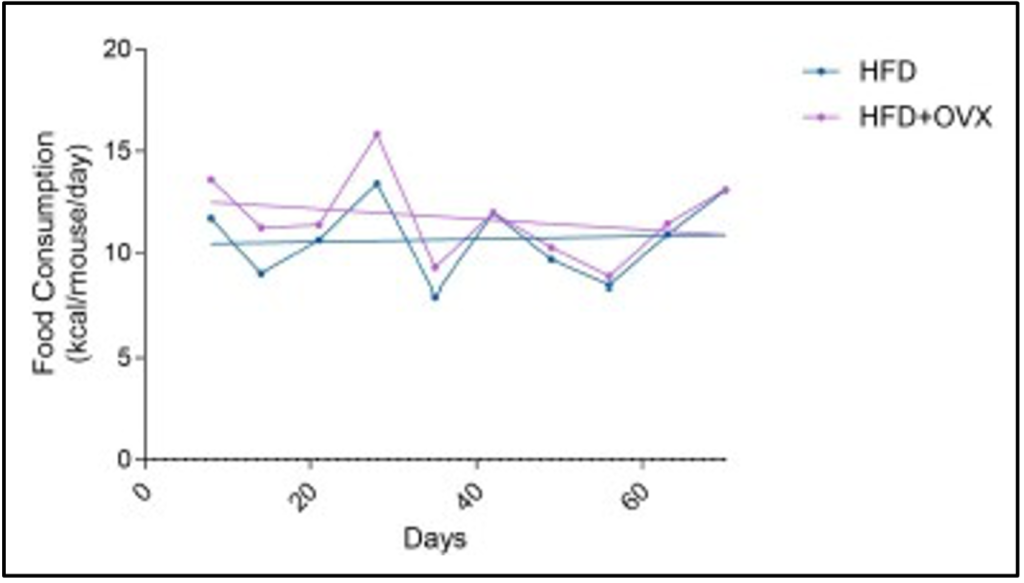
Figure 4. The kcal Consumption of the HFD Groups Over Time.
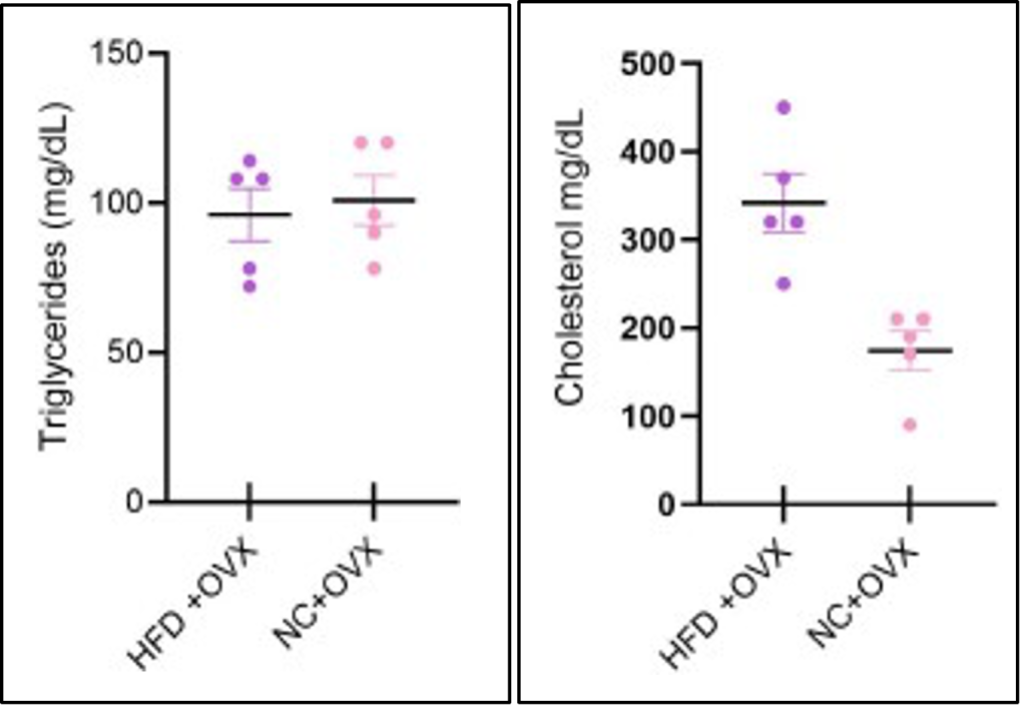
Figure 5. The Concentrations of Triglyceride and Cholesterol in the Blood Samples of the OVX Groups.
References
1. Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z., & Hall, M. E. (2015). Obesity- induced hypertension. Circulation Research, 116(6), 991–1006. https://doi.org/10.1161/circresaha.116.305697
2. Shaw, J. E., Sicree, R. A., & Zimmet, P. Z. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice, 87(1), 4–14. https:// doi.org/10.1016/j.diabres.2009.10.007
3. Lobo, R. A. (2008). Metabolic syndrome after menopause and the role of Hormones. Maturitas, 60(1), 10–18. https://doi.org/10.1016/j.maturitas.2008.02.008
4. Han, T. S., & Lean, M. E. J. (2015). Metabolic syndrome. Medicine, 43(2), 80–87. https://doi.org/10.1016/j.mpmed.2014.11.006
5. Brown, L. M., & Clegg, D. J. (2010). Central effects of estradiol in the regulation of food intake, body weight, and adiposity. The Journal of Steroid Biochemistry and Molecular Biology, 122(1–3), 65–73. https://doi.org/10.1016/j.jsbmb.2009.12.005
6. Ko, S.-H., & Kim, H.-S. (2020). Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients, 12(1), 202. https://doi.org/10.3390/nu12010202
7. Don Gambrell, R., & Teran, A.-Z. (1991). Changes in lipids and lipoproteins with long-term estrogen deficiency and hormone replacement therapy. American Journal of Obstetrics and Gynecology, 165(2), 307–317. https://doi.org/10.1016/0002-9378(91)90083-4
-
Lobo, R. A. (1995). Benefits and risks of Estrogen Replacement therapy. American Journal of Obstetrics and Gynecology, 173(3), 982–989. https://doi.org/10.1016/0002-9378(95)90247-3
- Woodruff, J. D., & Pickar, J. H. (1994). Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (premarin) with medroxyprogesterone acetate or conjugated estrogens alone. American Journal of Obstetrics and Gynecology, 170(5), 1213–1223. https://doi.org/10.1016/s0002-9378(94)70129-6
- Murrar, S., Johnson, P. A., Lee, Y.-G., & Carnes, M. (2021). Research conducted in women was deemed more impactful but less publishable than the same research conducted in men. Journal of Women’s Health, 30(9), 1259–1267. https://doi.org/10.1089/jwh.2020.8666
- Casimiro, I., Stull, N. D., Tersey, S. A., & Mirmira, R. G. (2021). Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J MICE. Journal of Diabetes and Its Complications, 35(2), 107795. https://doi.org/10.1016/j.jdiacomp.2020.107795
- Chaix, A., Deota, S., Bhardwaj, R., Lin, T., & Panda, S. (2021). Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a western high-fat high-sucrose diet in C57BL/6J MICE. Cell Reports, 36(7), 109543. https://doi.org/10.1016/j.celrep.2021.109543
- Pedroso, J., Camporez, J., Belpiede, L., Pinto, R., Cipolla-Neto, J., & Donato, J.(2019). Evaluation of hepatic steatosis in rodents by time-domain nuclear magnetic resonance. Diagnostics, 9(4), 198. https://doi.org/10.3390/diagnostics9040198

